Thrombolytic tPA-induced hemorrhagic transformation of ischemic stroke is mediated by PKCβ phosphorylation of occludin
- PMID: 35576527
- PMCID: PMC9335502
- DOI: 10.1182/blood.2021014958
Thrombolytic tPA-induced hemorrhagic transformation of ischemic stroke is mediated by PKCβ phosphorylation of occludin
Abstract
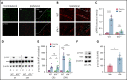
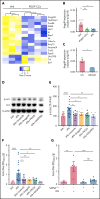
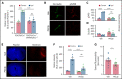
The current standard of care for moderate to severe ischemic stroke is thrombolytic therapy with tissue plasminogen activator (tPA). Treatment with tPA can significantly improve neurologic outcomes; however, thrombolytic therapy is associated with an increased risk of intracerebral hemorrhage (ICH). The risk of hemorrhage significantly limits the use of thrombolytic therapy, and identifying pathways induced by tPA that increase this risk could provide new therapeutic options to extend thrombolytic therapy to a wider patient population. Here, we investigate the role of protein kinase Cβ (PKCβ) phosphorylation of the tight junction protein occludin during ischemic stroke and its role in cerebrovascular permeability. We show that activation of this pathway by tPA is associated with an increased risk of ICH. Middle cerebral artery occlusion (MCAO) increased phosphorylation of occludin serine 490 (S490) in the ischemic penumbra in a tPA-dependent manner, as tPA-/- mice were significantly protected from MCAO-induced occludin phosphorylation. Intraventricular injection of tPA in the absence of ischemia was sufficient to induce occludin phosphorylation and vascular permeability in a PKCβ-dependent manner. Blocking occludin phosphorylation, either by targeted expression of a non-phosphorylatable form of occludin (S490A) or by pharmacologic inhibition of PKCβ, reduced MCAO-induced permeability and improved functional outcome. Furthermore, inhibiting PKCβ after MCAO prevented ICH associated with delayed thrombolysis. These results show that PKCβ phosphorylation of occludin is a downstream mediator of tPA-induced cerebrovascular permeability and suggest that PKCβ inhibitors could improve stroke outcome and prevent ICH associated with delayed thrombolysis, potentially extending the window for thrombolytic therapy in stroke.
© 2022 by The American Society of Hematology.
Figures
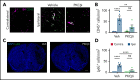
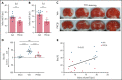
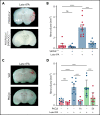
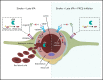
Comment in
-
Fibrinolysis without intracranial hemorrhage.Blood. 2022 Jul 28;140(4):300-302. doi: 10.1182/blood.2022016925. Blood. 2022. PMID: 35900787 No abstract available.
References
-
- Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1-122. - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. ; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. - PubMed
-
- Hacke W, Donnan G, Fieschi C, et al. ; NINDS rt-PA Study Group Investigators . Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004; 363(9411):768-774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

