Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine
- PMID: 35577151
- PMCID: PMC9121740
- DOI: 10.1016/j.jconrel.2022.05.023
Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine
Abstract
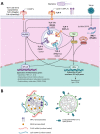
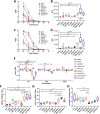
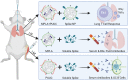
Despite success in vaccinating populations against SARS-CoV-2, concerns about immunity duration, continued efficacy against emerging variants, protection from infection and transmission, and worldwide vaccine availability remain. Molecular adjuvants targeting pattern recognition receptors (PRRs) on antigen-presenting cells (APCs) could improve and broaden the efficacy and durability of vaccine responses. Native SARS-CoV-2 infection stimulates various PRRs, including toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors. We hypothesized that targeting PRRs using molecular adjuvants on nanoparticles (NPs) along with a stabilized spike protein antigen could stimulate broad and efficient immune responses. Adjuvants targeting TLR4 (MPLA), TLR7/8 (R848), TLR9 (CpG), and RIG-I (PUUC) delivered on degradable polymer NPs were combined with the S1 subunit of spike protein and assessed in vitro with isogeneic mixed lymphocyte reactions (isoMLRs). For in vivo studies, the adjuvant-NPs were combined with stabilized spike protein or spike-conjugated NPs and assessed using a two-dose intranasal or intramuscular vaccination model in mice. Combination adjuvant-NPs simultaneously targeting TLR and RIG-I receptors (MPLA+PUUC, CpG+PUUC, and R848+PUUC) differentially induced T cell proliferation and increased proinflammatory cytokine secretion by APCs in vitro. When delivered intranasally, MPLA+PUUC NPs enhanced CD4+CD44+ activated memory T cell responses against spike protein in the lungs while MPLA NPs increased anti-spike IgA in the bronchoalveolar (BAL) fluid and IgG in the blood. Following intramuscular delivery, PUUC NPs induced strong humoral immune responses, characterized by increases in anti-spike IgG in the blood and germinal center B cell populations (GL7+ and BCL6+ B cells) in the draining lymph nodes (dLNs). MPLA+PUUC NPs further boosted spike protein-neutralizing antibody titers and T follicular helper cell populations in the dLNs. These results suggest that protein subunit vaccines with particle-delivered molecular adjuvants targeting TLR4 and RIG-I could lead to robust and unique route-specific adaptive immune responses against SARS-CoV-2.
Keywords: Adaptive immune response; COVID-19 protein subunit vaccine; Combination adjuvant; Intranasal versus intramuscular vaccination; Monophosphoryl lipid A; SARS-CoV-2 spike protein.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose.
Figures



Update of
-
Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine.bioRxiv [Preprint]. 2022 Feb 2:2022.01.31.478507. doi: 10.1101/2022.01.31.478507. bioRxiv. 2022. Update in: J Control Release. 2022 Jul;347:476-488. doi: 10.1016/j.jconrel.2022.05.023. PMID: 35132413 Free PMC article. Updated. Preprint.
References
-
- R&D Blue Print . 2021. COVID-19 Vaccine Tracker and Landscape.
-
- Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

