SARS-CoV-2 saliva testing using RT-PCR: a systematic review
- PMID: 35577250
- PMCID: PMC9136484
- DOI: 10.1016/j.ijid.2022.05.008
SARS-CoV-2 saliva testing using RT-PCR: a systematic review
Abstract
Objectives: There remain challenges in using SARS-CoV-2 RNA diagnostic assays in the respiratory tract in a pandemic. More so certain countries such as Hong Kong have already included saliva as part of their mass-testing protocol. The aim of this study was to conduct a systematic review on the alternate use of saliva as a SARS-CoV-2 RNA testing specimen in the context of mass screening with reverse transcription polymerase chain reaction.
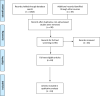
Methods: Our search methodology was modeled after the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist, and the risk of bias of the selected studies was qualitatively assessed. The percentage individual positive and percentage agreement of both index (saliva) and reference (nasopharyngeal swab), in preference to specificity and sensitivity, were estimated using Kappa statistics.
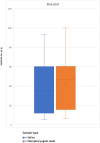
Results: A total of 44 studies met the inclusion criteria. The average percentage positive saliva cases was 72.7% (95% confidence interval), which was lower but not substantially different from the percentage positive NPS of 78.7% (95% confidence interval), and there was an average overall agreement of 89.7% (95% confidence interval).
Conclusion: Although the literature supports nasopharyngeal swab as a superior testing specimen, an alternative clinical specimen in saliva may offer potential benefits such that a potentially reduced accuracy may be tolerated, especially in low socioeconomic regions.
Keywords: Covid-19; Nasopharyngeal swab; RT-PCR; SARS-CoV-2; Saliva.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest The authors have no competing interests to declare.
Figures
Similar articles
-
Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru.Microbiol Spectr. 2022 Aug 31;10(4):e0086122. doi: 10.1128/spectrum.00861-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867471 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Diagnostic Performance and Tolerability of Saliva and Nasopharyngeal Swab Specimens in the Detection of SARS-CoV-2 by RT-PCR.Microbiol Spectr. 2023 Jun 15;11(3):e0532422. doi: 10.1128/spectrum.05324-22. Epub 2023 Apr 24. Microbiol Spectr. 2023. PMID: 37093085 Free PMC article.
-
Alternative clinical specimens for the detection of SARS-CoV-2: A rapid review.Rev Med Virol. 2021 Jul;31(4):e2185. doi: 10.1002/rmv.2185. Epub 2020 Oct 22. Rev Med Virol. 2021. PMID: 33091200 Review.
-
Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature.Ear Nose Throat J. 2021 Apr;100(2_suppl):131S-138S. doi: 10.1177/0145561320953231. Epub 2020 Aug 31. Ear Nose Throat J. 2021. PMID: 32865458 Free PMC article. Review.
Cited by
-
Cross comparison of alternative diagnostic protocols including substitution to the clinical sample, RNA extraction method and nucleic acid amplification technology for COVID-19 diagnosis.Front Mol Biosci. 2024 Aug 23;11:1445142. doi: 10.3389/fmolb.2024.1445142. eCollection 2024. Front Mol Biosci. 2024. PMID: 39247206 Free PMC article.
-
Optimization and application of digital droplet PCR for the detection of SARS-CoV-2 in saliva specimen using commercially available kit.Biol Methods Protoc. 2024 Sep 23;9(1):bpae068. doi: 10.1093/biomethods/bpae068. eCollection 2024. Biol Methods Protoc. 2024. PMID: 39355137 Free PMC article.
-
Kinetics of SARS-CoV-2 infection biomarkers in a household transmission study.Sci Rep. 2024 May 29;14(1):12365. doi: 10.1038/s41598-024-62835-0. Sci Rep. 2024. PMID: 38811590 Free PMC article.
-
Recent advances in RNA sample preparation techniques for the detection of SARS-CoV-2 in saliva and gargle.Trends Analyt Chem. 2023 Aug;165:117107. doi: 10.1016/j.trac.2023.117107. Epub 2023 May 23. Trends Analyt Chem. 2023. PMID: 37317683 Free PMC article. Review.
-
The role of the oral cavity in SARS-CoV-2- and other viral infections.Clin Oral Investig. 2023 Jun;27(Suppl 1):15-22. doi: 10.1007/s00784-023-05078-z. Epub 2023 Jun 13. Clin Oral Investig. 2023. PMID: 37310513 Free PMC article. Review.
References
-
- Azzi L, Baj A, Alberio T, Lualdi M, Veronesi G, Carcano G, Ageno W, Gambarini C, Maffioli L, Saverio SD, Gasperina DD, Genoni AP, Premi E, Donati S, Azzolini C, Grandi AM, Dentali F, Tangianu F, Sessa F, Maurino V, Tettamanti L, Siracusa C, Vigezzi A, Monti E, Iori V, Iovino D, Ietto G, Group ADSLRSTNSR, Grossi P.A., Tagliabue A., Fasano M. Rapid Salivary Test suitable for a mass screening program to detect SARS-CoV-2: a diagnostic accuracy study. J Infect. 2020;81:e75–e78. - PMC - PubMed
-
- Bhattacharya D, Parai D, Rout UK, Dash P, Nanda RR, Dash GC, Kanungo S, Palo SK, Giri S, Choudhary HR, Kshatri JS, Turuk J, Mishra BK, Lenka RK, Dash S, Pati S. Saliva for diagnosis of SARS-CoV-2: first report from India. J Med Virol. 2021;93:2529–2533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous