Telehealth cancer-related fatigue clinic model for cancer survivors: a pilot randomised controlled trial protocol (the T-CRF trial)
- PMID: 35577469
- PMCID: PMC9114967
- DOI: 10.1136/bmjopen-2021-059952
Telehealth cancer-related fatigue clinic model for cancer survivors: a pilot randomised controlled trial protocol (the T-CRF trial)
Abstract
Introduction: Cancer-related fatigue (CRF) is one of the most common and debilitating adverse effects of cancer and its treatment reported by cancer survivors. Physical activity, psychological interventions and management of concurrent symptoms have been shown to be effective in alleviating CRF. This pilot randomised controlled trial (RCT) will determine the feasibility of a telehealth CRF clinic intervention (T-CRF) to implement evidence-based strategies and assess the impact of the intervention on CRF and other clinical factors in comparison to usual care.
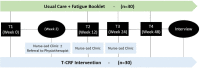
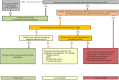
Methods and analysis: A parallel-arm (intervention vs usual care) pilot RCT will be conducted at the Princess Alexandra Hospital in Queensland, Australia. Sixty cancer survivors aged 18 years and over, who report moderate or severe fatigue on the Brief Fatigue Inventory and meet other study criteria will be recruited. Participants will be randomised (1:1) to receive the T-CRF intervention or usual care (ie, specialist-led care, with a fatigue information booklet). The intervention is a 24-week programme of three telehealth nurse-led consultations and a personalised CRF management plan. The primary objective of this pilot RCT is to determine intervention feasibility, with a secondary objective to determine preliminary clinical efficacy. Feasibility outcomes include the identification of recruitment methods; recruitment rate and uptake; attrition; adherence; fidelity; apathy; and intervention functionality, acceptability and satisfaction. Clinical and resource use outcomes include cancer survivor fatigue, symptom burden, level of physical activity, productivity loss, hospital resource utilisation and carer's fatigue and productivity loss. Descriptive statistics will be used to report on feasibility and process-related elements additional to clinical and resource outcomes.
Ethics and dissemination: This trial is prospectively registered (ACTRN12620001334998). The study protocol has been approved by the Metro South Health and Hospital Services Human Research Ethics Committee (MSHHS HREC/2020/QMS/63495). Findings will be disseminated through peer-reviewed publications, national and international conferences and seminars or workshops.
Trial registration number: Australian New Zealand Clinical Trials Registry ID: ACTRN12620001334998; Pre-results. Trial Version: Version 1.1. Last updated 10 December 2020.
Keywords: COMPLEMENTARY MEDICINE; ONCOLOGY; Telemedicine.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Protocol for a pilot randomised controlled trial of an online intervention for post-treatment cancer survivors with persistent fatigue.BMJ Open. 2016 Jun 10;6(6):e011485. doi: 10.1136/bmjopen-2016-011485. BMJ Open. 2016. PMID: 27288384 Free PMC article. Clinical Trial.
-
i-Move, a personalised exercise intervention for patients with advanced melanoma receiving immunotherapy: a randomised feasibility trial protocol.BMJ Open. 2020 Feb 28;10(2):e036059. doi: 10.1136/bmjopen-2019-036059. BMJ Open. 2020. PMID: 32114479 Free PMC article. Clinical Trial.
-
Safety and adherence to medications and self-care advice in oncology (SAMSON): pilot randomised controlled trial protocol.BMJ Open. 2024 Jul 23;14(7):e079122. doi: 10.1136/bmjopen-2023-079122. BMJ Open. 2024. PMID: 39043598 Free PMC article.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
-
The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: a systematic review.J Cancer Surviv. 2019 Feb;13(1):97-107. doi: 10.1007/s11764-018-0730-8. Epub 2019 Jan 11. J Cancer Surviv. 2019. PMID: 30635865
Cited by
-
Identifying strategies for implementing a clinical guideline for cancer-related fatigue: a qualitative study.BMC Health Serv Res. 2023 Apr 24;23(1):395. doi: 10.1186/s12913-023-09377-9. BMC Health Serv Res. 2023. PMID: 37095506 Free PMC article.
-
Health Care Contact Days Among Older Cancer Survivors.JCO Oncol Pract. 2024 Jul;20(7):943-952. doi: 10.1200/OP.23.00590. Epub 2024 Mar 7. JCO Oncol Pract. 2024. PMID: 38452315 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
