Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF
- PMID: 35577554
- PMCID: PMC9186798
- DOI: 10.1523/JNEUROSCI.2273-21.2022
Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF
Abstract
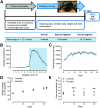
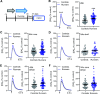
Physical exercise improves motor performance in individuals with Parkinson's disease and elevates mood in those with depression. Although underlying factors have not been identified, clues arise from previous studies showing a link between cognitive benefits of exercise and increases in brain-derived neurotrophic factor (BDNF). Here, we investigated the influence of voluntary wheel-running exercise on BDNF levels in the striatum of young male wild-type (WT) mice, and on the striatal release of a key motor-system transmitter, dopamine (DA). Mice were allowed unlimited access to a freely rotating wheel (runners) or a locked wheel (controls) for 30 d. Electrically evoked DA release was quantified in ex vivo corticostriatal slices from these animals using fast-scan cyclic voltammetry. We found that exercise increased BDNF levels in dorsal striatum (dStr) and increased DA release in dStr and in nucleus accumbens core and shell. Increased DA release was independent of striatal acetylcholine (ACh), and persisted after a week of rest. We tested a role for BDNF in the influence of exercise on DA release using mice that were heterozygous for BDNF deletion (BDNF+/-). In contrast to WT mice, evoked DA release did not differ between BDNF+/- runners and controls. Complementary pharmacological studies using a tropomyosin receptor kinase B (TrkB) agonist in WT mouse slices showed that TrkB receptor activation also increased evoked DA release throughout striatum in an ACh-independent manner. Together, these data support a causal role for BDNF in exercise-enhanced striatal DA release and provide mechanistic insight into the beneficial effects of exercise in neuropsychiatric disorders, including Parkinson's, depression, and anxiety.SIGNIFICANCE STATEMENT Exercise has been shown to improve movement and cognition in humans and rodents. Here, we report that voluntary exercise for 30 d leads to an increase in evoked DA release throughout the striatum and an increase in BDNF in the dorsal (motor) striatum. The increase in DA release appears to require BDNF, indicated by the absence of DA release enhancement with running in BDNF+/- mice. Activation of BDNF receptors using a pharmacological agonist was also shown to boost DA release. Together, these data support a necessary and sufficient role for BDNF in exercise-enhanced DA release and provide mechanistic insight into the reported benefits of exercise in individuals with dopamine-linked neuropsychiatric disorders, including Parkinson's disease and depression.
Keywords: Parkinson's disease; brain slices; fast-scan cyclic voltammetry; nAChRs; nucleus accumbens; running wheel.
Copyright © 2022 the authors.
Figures





References
-
- Aguiar AS Jr, Tristão FS, Amar M, Chevarin C, Glaser V, de Paula Martins R, Moreira EL, Mongeau R, Lanfumey L, Raisman-Vozari R, Latini A, Prediger RD (2014) Six weeks of voluntary exercise don't protect C57BL/6 mice against neurotoxicity of MPTP and MPP+. Neurotox Res 25:147–152. 10.1007/s12640-013-9412-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
