An Artificial Intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease
- PMID: 35577777
- PMCID: PMC9110726
- DOI: 10.1038/s41467-022-30357-w
An Artificial Intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease
Erratum in
-
Publisher Correction: An Artificial Intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease.Nat Commun. 2022 Aug 11;13(1):4729. doi: 10.1038/s41467-022-32479-7. Nat Commun. 2022. PMID: 35953486 Free PMC article. No abstract available.
Abstract
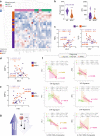
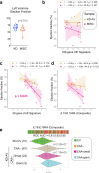
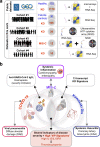
Multisystem inflammatory syndrome in children (MIS-C) is an illness that emerged amidst the COVID-19 pandemic but shares many clinical features with the pre-pandemic syndrome of Kawasaki disease (KD). Here we compare the two syndromes using a computational toolbox of two gene signatures that were developed in the context of SARS-CoV-2 infection, i.e., the viral pandemic (ViP) and severe-ViP signatures and a 13-transcript signature previously demonstrated to be diagnostic for KD, and validated our findings in whole blood RNA sequences, serum cytokines, and formalin fixed heart tissues. Results show that KD and MIS-C are on the same continuum of the host immune response as COVID-19. Both the pediatric syndromes converge upon an IL15/IL15RA-centric cytokine storm, suggestive of shared proximal pathways of immunopathogenesis; however, they diverge in other laboratory parameters and cardiac phenotypes. The ViP signatures reveal unique targetable cytokine pathways in MIS-C, place MIS-C farther along in the spectrum in severity compared to KD and pinpoint key clinical (reduced cardiac function) and laboratory (thrombocytopenia and eosinopenia) parameters that can be useful to monitor severity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Kawasaki disease and MIS-C share a host immune response.Nat Rev Rheumatol. 2022 Oct;18(10):555-556. doi: 10.1038/s41584-022-00820-5. Nat Rev Rheumatol. 2022. PMID: 36008614 Free PMC article.
Similar articles
-
An AI-guided signature reveals the nature of the shared proximal pathways of host immune response in MIS-C and Kawasaki disease.bioRxiv [Preprint]. 2021 Aug 9:2021.04.11.439347. doi: 10.1101/2021.04.11.439347. bioRxiv. 2021. PMID: 33880476 Free PMC article. Preprint.
-
Phenotype, Susceptibility, Autoimmunity, and Immunotherapy Between Kawasaki Disease and Coronavirus Disease-19 Associated Multisystem Inflammatory Syndrome in Children.Front Immunol. 2021 Feb 26;12:632890. doi: 10.3389/fimmu.2021.632890. eCollection 2021. Front Immunol. 2021. PMID: 33732254 Free PMC article.
-
Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease.J Clin Invest. 2021 Mar 15;131(6):e144554. doi: 10.1172/JCI144554. J Clin Invest. 2021. PMID: 33497356 Free PMC article.
-
Current Understanding of Multisystem Inflammatory Syndrome (MIS-C) Following COVID-19 and Its Distinction from Kawasaki Disease.Curr Rheumatol Rep. 2021 Jul 3;23(8):58. doi: 10.1007/s11926-021-01028-4. Curr Rheumatol Rep. 2021. PMID: 34216296 Free PMC article. Review.
-
RNAseq-based transcriptomics of treatment-naïve multi-inflammatory syndrome in children (MIS-C) demonstrates predominant activation of matrisome, innate and humoral immune pathways.Rheumatol Int. 2024 Aug;44(8):1445-1454. doi: 10.1007/s00296-023-05478-0. Epub 2023 Oct 12. Rheumatol Int. 2024. PMID: 37823896
Cited by
-
Comparable bidirectional neutrophil immune dysregulation between Kawasaki disease and severe COVID-19.Front Immunol. 2022 Sep 8;13:995886. doi: 10.3389/fimmu.2022.995886. eCollection 2022. Front Immunol. 2022. PMID: 36159873 Free PMC article.
-
Transcriptome meta-analysis of Kawasaki disease in humans and mice.Front Pediatr. 2024 Sep 16;12:1423958. doi: 10.3389/fped.2024.1423958. eCollection 2024. Front Pediatr. 2024. PMID: 39350793 Free PMC article.
-
Tolerability of COVID-19 Infection and Messenger RNA Vaccination Among Patients With a History of Kawasaki Disease.JAMA Netw Open. 2022 Aug 1;5(8):e2226236. doi: 10.1001/jamanetworkopen.2022.26236. JAMA Netw Open. 2022. PMID: 35960521 Free PMC article.
-
Differentiating Multisystem Inflammatory Syndrome in Children from Kawasaki Disease During the Pandemic.Turk Arch Pediatr. 2024 Mar;59(2):150-156. doi: 10.5152/TurkArchPediatr.2024.23192. Turk Arch Pediatr. 2024. PMID: 38454223 Free PMC article.
-
Kawasaki Disease-Associated Cytokine Storm Syndrome.Adv Exp Med Biol. 2024;1448:365-383. doi: 10.1007/978-3-031-59815-9_25. Adv Exp Med Biol. 2024. PMID: 39117827 Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

