Ca2+-mediated higher-order assembly of heterodimers in amino acid transport system b0,+ biogenesis and cystinuria
- PMID: 35577790
- PMCID: PMC9110406
- DOI: 10.1038/s41467-022-30293-9
Ca2+-mediated higher-order assembly of heterodimers in amino acid transport system b0,+ biogenesis and cystinuria
Abstract
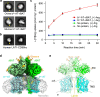
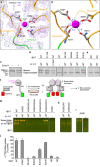
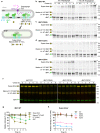
Cystinuria is a genetic disorder characterized by overexcretion of dibasic amino acids and cystine, causing recurrent kidney stones and kidney failure. Mutations of the regulatory glycoprotein rBAT and the amino acid transporter b0,+AT, which constitute system b0,+, are linked to type I and non-type I cystinuria respectively and they exhibit distinct phenotypes due to protein trafficking defects or catalytic inactivation. Here, using electron cryo-microscopy and biochemistry, we discover that Ca2+ mediates higher-order assembly of system b0,+. Ca2+ stabilizes the interface between two rBAT molecules, leading to super-dimerization of b0,+AT-rBAT, which in turn facilitates N-glycan maturation and protein trafficking. A cystinuria mutant T216M and mutations of the Ca2+ site of rBAT cause the loss of higher-order assemblies, resulting in protein trapping at the ER and the loss of function. These results provide the molecular basis of system b0,+ biogenesis and type I cystinuria and serve as a guide to develop new therapeutic strategies against it. More broadly, our findings reveal an unprecedented link between transporter oligomeric assembly and protein-trafficking diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Comment in
-
Uro-Science.J Urol. 2023 Jan;209(1):289-291. doi: 10.1097/JU.0000000000003033. Epub 2022 Oct 21. J Urol. 2023. PMID: 36268621 No abstract available.
-
Urolithiasis/Endourology.J Urol. 2023 May;209(5):1021-1023. doi: 10.1097/JU.0000000000003379. Epub 2023 Feb 22. J Urol. 2023. PMID: 37026651 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

