A metamodel-based flexible insulin therapy for type 1 diabetes patients subjected to aerobic physical activity
- PMID: 35577814
- PMCID: PMC9110411
- DOI: 10.1038/s41598-022-11772-x
A metamodel-based flexible insulin therapy for type 1 diabetes patients subjected to aerobic physical activity
Abstract
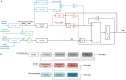
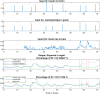
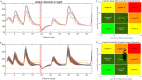
Patients with type 1 diabetes are subject to exogenous insulin injections, whether manually or through (semi)automated insulin pumps. Basic knowledge of the patient's characteristics and flexible insulin therapy (FIT) parameters are then needed. Specifically, artificial pancreas-like closed-loop insulin delivery systems are some of the most promising devices for substituting for endogenous insulin secretion in type 1 diabetes patients. However, these devices require self-reported information such as carbohydrates or physical activity from the patient, introducing potential miscalculations and delays that can have life-threatening consequences. Here, we display a metamodel for glucose-insulin dynamics that is subject to carbohydrate ingestion and aerobic physical activity. This metamodel incorporates major existing knowledge-based models. We derive comprehensive and universal definitions of the underlying FIT parameters to form an insulin sensitivity factor (ISF). In addition, the relevance of physical activity modelling is assessed, and the FIT is updated to take physical exercise into account. Specifically, we cope with physical activity by using heart rate sensors (watches) with a fully automated closed insulin loop, aiming to maximize the time spent in the glycaemic range (75.5% in the range and 1.3% below the range for hypoglycaemia on a virtual patient simulator).These mathematical parameter definitions are interesting on their own, may be new tools for assessing mathematical models and can ultimately be used in closed-loop artificial pancreas algorithms or to extend distinguished FIT.
Trial registration: ClinicalTrials.gov NCT04572009.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: a single-centre, open-label, randomised controlled, crossover, non-inferiority trial.Lancet Digit Health. 2021 Nov;3(11):e723-e732. doi: 10.1016/S2589-7500(21)00139-4. Epub 2021 Sep 24. Lancet Digit Health. 2021. PMID: 34580055 Clinical Trial.
-
Automated insulin pump suspension for hypoglycaemia mitigation: development, implementation and implications.Diabetes Obes Metab. 2015 Dec;17(12):1126-32. doi: 10.1111/dom.12542. Epub 2015 Oct 5. Diabetes Obes Metab. 2015. PMID: 26179879 Review.
-
Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study.Diabetes Obes Metab. 2017 Dec;19(12):1698-1705. doi: 10.1111/dom.12999. Epub 2017 Jul 6. Diabetes Obes Metab. 2017. PMID: 28474383 Free PMC article.
-
[What is the current state of the artificial pancreas in diabetes care?].Internist (Berl). 2020 Jan;61(1):102-109. doi: 10.1007/s00108-019-00713-y. Internist (Berl). 2020. PMID: 31863132 Review. German.
-
Dynamic Insulin Basal Needs Estimation and Parameters Adjustment in Type 1 Diabetes.Sensors (Basel). 2021 Aug 2;21(15):5226. doi: 10.3390/s21155226. Sensors (Basel). 2021. PMID: 34372462 Free PMC article.
References
-
- Weisman A, Bai J-W, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501–512. doi: 10.1016/S2213-8587(17)30167-5. - DOI - PubMed
-
- Brooker, G. Handbook of Biomechatronics, chap. 11-The Artificial Pancreas (Academic Press, 2019).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

