RT-qPCR-based tests for SARS-CoV-2 detection in pooled saliva samples for massive population screening to monitor epidemics
- PMID: 35577836
- PMCID: PMC9109753
- DOI: 10.1038/s41598-022-12179-4
RT-qPCR-based tests for SARS-CoV-2 detection in pooled saliva samples for massive population screening to monitor epidemics
Abstract
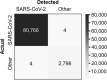
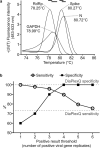
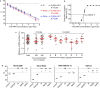
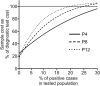
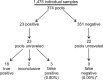
Swab, RT-qPCR tests remain the gold standard of diagnostics of SARS-CoV-2 infections. These tests are costly and have limited throughput. We developed a 3-gene, seminested RT-qPCR test with SYBR green-based detection designed to be oversensitive rather than overspecific for high-throughput diagnostics of populations. This two-tier approach depends on decentralized self-collection of saliva samples, pooling, 1st-tier testing with highly sensitive screening test and subsequent 2nd-tier testing of individual samples from positive pools with the IVD test. The screening test was able to detect five copies of the viral genome in 10 µl of isolated RNA with 50% probability and 18.8 copies with 95% probability and reached Ct values that were highly linearly RNA concentration-dependent. In the side-by-side comparison, the screening test attained slightly better results than the commercially available IVD-certified RT-qPCR diagnostic test DiaPlexQ (100% specificity and 89.8% sensitivity vs. 100% and 73.5%, respectively). Testing of 1475 individual clinical samples pooled in 374 pools of four revealed 0.8% false positive pools and no false negative pools. In weekly prophylactic testing of 113 people within 6 months, a two-tier testing approach enabled the detection of 18 infected individuals, including several asymptomatic individuals, with substantially lower cost than individual RT-PCR testing.
© 2022. The Author(s).
Conflict of interest statement
Jarosław Dastych is the CEO of Proteon Pharmaceuticals S.A., which offers the presented method as a commercial service. The other authors declare no competing interests.
Figures
References
-
- WHO. COVID-19 Weekly Epidemiological Update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Access 20 Apr 2021 (2021).
-
- WHO. COVID-19 Weekly Epidemiological Update. https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... Access 21 Mar 2022 (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

