In vivo imaging with a fast large-area multiphoton exoscope (FLAME) captures the melanin distribution heterogeneity in human skin
- PMID: 35577848
- PMCID: PMC9110384
- DOI: 10.1038/s41598-022-12317-y
In vivo imaging with a fast large-area multiphoton exoscope (FLAME) captures the melanin distribution heterogeneity in human skin
Abstract
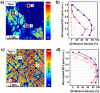
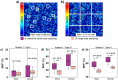
Melanin plays a significant role in the regulation of epidermal homeostasis and photoprotection of human skin. The assessment of its epidermal distribution and overall content is of great interest due to its involvement in a wide range of physiological and pathological skin processes. Among several spectroscopic and optical imaging methods that have been reported for non-invasive quantification of melanin in human skin, the approach based on the detection of two-photon excited fluorescence lifetime distinguishes itself by enabling selective detection of melanin with sub-cellular resolution, thus facilitating its quantification while also resolving its depth-profile. A key limitation of prior studies on the melanin assessment based on this approach is their inability to account for the skin heterogeneity due to the reduced field of view of the images, which results in high dispersion of the measurement values. Pigmentation in both normal and pathological human skin is highly heterogeneous and its macroscopic quantification is critical for reliable measurements of the epidermal melanin distribution and for capturing melanin-related sensitive dynamic changes as a response to treatment. In this work, we employ a fast large-area multiphoton exoscope (FLAME), recently developed by our group for clinical skin imaging, that has the ability to evaluate the 3D distribution of epidermal melanin content in vivo macroscopically (millimeter scale) with microscopic resolution (sub-micron) and rapid acquisition rates (minutes). We demonstrate significant enhancement in the reliability of the melanin density and distribution measurements across Fitzpatrick skin types I to V by capturing the intra-subject pigmentation heterogeneity enabled by the large volumetric sampling. We also demonstrate the potential of this approach to provide consistent measurement results when imaging the same skin area at different times. These advances are critical for clinical and research applications related to monitoring pigment modulation as a response to therapies against pigmentary skin disorders, skin aging, as well as skin cancers.
© 2022. The Author(s).
Conflict of interest statement
M. Balu is co-author of a patent owned by the University of California, Irvine, which is related to the MPM imaging technology. M. Balu is also a co-founder of Infraderm, LLC, a startup spin off from UC Irvine, that develops MPM-based clinical imaging platforms for commercialization purpose. The Institutional Review Board and Conflict of Interest Office of the University of California, Irvine, have reviewed these disclosures and did not find any concerns. The other authors disclosed no conflicts of interest.
Figures



Similar articles
-
Fast, large area multiphoton exoscope (FLAME) for macroscopic imaging with microscopic resolution of human skin.Sci Rep. 2020 Oct 22;10(1):18093. doi: 10.1038/s41598-020-75172-9. Sci Rep. 2020. PMID: 33093610 Free PMC article.
-
In vivo melanin 3D quantification and z-epidermal distribution by multiphoton FLIM, phasor and Pseudo-FLIM analyses.Sci Rep. 2022 Jan 31;12(1):1642. doi: 10.1038/s41598-021-03114-0. Sci Rep. 2022. PMID: 35102172 Free PMC article.
-
In vivo multiphoton imaging for non-invasive time course assessment of retinoids effects on human skin.Skin Res Technol. 2020 Nov;26(6):794-803. doi: 10.1111/srt.12877. Epub 2020 Jul 26. Skin Res Technol. 2020. PMID: 32713074 Free PMC article.
-
[Normal and abnormal skin color].Ann Dermatol Venereol. 2012 Nov;139 Suppl 3:S73-7. doi: 10.1016/S0151-9638(12)70114-X. Ann Dermatol Venereol. 2012. PMID: 23260521 Review. French.
-
Normal and abnormal skin color.Ann Dermatol Venereol. 2012 Dec;139 Suppl 4:S125-9. doi: 10.1016/S0151-9638(12)70123-0. Ann Dermatol Venereol. 2012. PMID: 23522626 Review.
Cited by
-
Motion-tolerant 3D volumetric multimodality microscopy imaging of human skin with subcellular resolution and extended field-of-view.Commun Biol. 2025 Feb 5;8(1):186. doi: 10.1038/s42003-025-07614-x. Commun Biol. 2025. PMID: 39910179 Free PMC article.
-
In vivo multiphoton multiparametric 3D quantification of human skin aging on forearm and face.Sci Rep. 2022 Sep 1;12(1):14863. doi: 10.1038/s41598-022-18657-z. Sci Rep. 2022. PMID: 36050338 Free PMC article.
-
Intraoperative microscopic autofluorescence detection and characterization in brain tumors using stimulated Raman histology and two-photon fluorescence.Front Oncol. 2023 May 10;13:1146031. doi: 10.3389/fonc.2023.1146031. eCollection 2023. Front Oncol. 2023. PMID: 37234975 Free PMC article.
-
A 20 MHz Repetition Rate, Sub-Picosecond Ti-Sapphire Laser for Fiber Delivery in Nonlinear Microscopy of the Skin.Life (Basel). 2024 Feb 7;14(2):231. doi: 10.3390/life14020231. Life (Basel). 2024. PMID: 38398740 Free PMC article.
-
Global research trends on melasma: a bibliometric and visualized study from 2014 to 2023.Front Pharmacol. 2024 Jul 25;15:1421499. doi: 10.3389/fphar.2024.1421499. eCollection 2024. Front Pharmacol. 2024. PMID: 39119611 Free PMC article. Review.
References
-
- Watt AAR, Bothma JP, Meredith P. The supramolecular structure of melanin. Soft Matter. 2009;5:3754. doi: 10.1039/b902507c. - DOI

