Muscle contractions and pain sensation accompanying high-frequency electroporation pulses
- PMID: 35577873
- PMCID: PMC9110404
- DOI: 10.1038/s41598-022-12112-9
Muscle contractions and pain sensation accompanying high-frequency electroporation pulses
Abstract
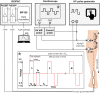
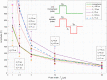
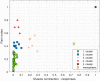
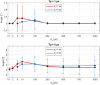
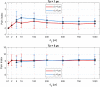
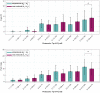
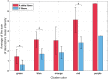
To minimize neuromuscular electrical stimulation during electroporation-based treatments, the replacement of long monophasic pulses with bursts of biphasic high-frequency pulses in the range of microseconds was suggested in order to reduce muscle contraction and pain sensation due to pulse application. This treatment modality appeared under the term high-frequency electroporation (HF-EP), which can be potentially used for some clinical applications of electroporation such as electrochemotherapy, gene electrotransfer, and tissue ablation. In cardiac tissue ablation, which utilizes irreversible electroporation, the treatment is being established as Pulsed Field Ablation. While the reduction of muscle contractions was confirmed in multiple in vivo studies, the reduction of pain sensation in humans was not confirmed yet, nor was the relationship between muscle contraction and pain sensation investigated. This is the first study in humans examining pain sensation using biphasic high-frequency electroporation pulses. Twenty-five healthy individuals were subjected to electrical stimulation of the tibialis anterior muscle with biphasic high-frequency pulses in the range of few microseconds and both, symmetric and asymmetric interphase and interpulse delays. Our results confirm that biphasic high-frequency pulses with a pulse width of 1 or 2 µs reduce muscle contraction and pain sensation as opposed to currently used longer monophasic pulses. In addition, interphase and interpulse delays play a significant role in reducing the muscle contraction and/or pain sensation. The study shows that the range of the optimal pulse parameters may be increased depending on the prerequisites of the therapy. However, further evaluation of the biphasic pulse protocols presented herein is necessary to confirm the efficiency of the newly proposed HF-EP.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Batista NT, Polajžer T, Miklavčič D. Cell death due to electroporation–a review. Bioelectrochemistry. 2021;141:1150. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

