Complex tools and motor-to-mechanical transformations
- PMID: 35577883
- PMCID: PMC9110343
- DOI: 10.1038/s41598-022-12142-3
Complex tools and motor-to-mechanical transformations
Abstract
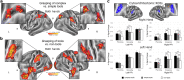
The ability to use complex tools is thought to depend on multifaceted motor-to-mechanical transformations within the left inferior parietal lobule (IPL), linked to cognitive control over compound actions. Here we show using neuroimaging that demanding transformations of finger movements into proper mechanical movements of functional parts of complex tools invoke significantly the right rather than left rostral IPL, and bilateral posterior-to-mid and left anterior intraparietal sulci. These findings emerged during the functional grasp and tool-use programming phase. The expected engagement of left IPL was partly revealed by traditional region-of-interest analyses, and further modeling/estimations at the hand-independent level. Thus, our results point to a special role of right IPL in supporting sensory-motor spatial mechanisms which enable an effective control of fingers in skillful handling of complex tools. The resulting motor-to-mechanical transformations involve dynamic hand-centered to target-centered reference frame conversions indispensable for efficient interactions with the environment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chevalier-Skolnikoff S. Spontaneous tool use and sensorimotor intelligence in Cebus compared with other monkeys and apes. Behav. Brain Sci. 1989;12:561–627. doi: 10.1017/S0140525X00057678. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

