Ciliary central apparatus structure reveals mechanisms of microtubule patterning
- PMID: 35578023
- PMCID: PMC9930914
- DOI: 10.1038/s41594-022-00770-2
Ciliary central apparatus structure reveals mechanisms of microtubule patterning
Abstract
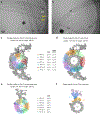
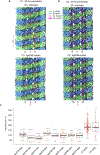
A pair of extensively modified microtubules form the central apparatus (CA) of the axoneme of most motile cilia, where they regulate ciliary motility. The external surfaces of both CA microtubules are patterned asymmetrically with large protein complexes that repeat every 16 or 32 nm. The composition of these projections and the mechanisms that establish asymmetry and longitudinal periodicity are unknown. Here, by determining cryo-EM structures of the CA microtubules, we identify 48 different CA-associated proteins, which in turn reveal mechanisms for asymmetric and periodic protein binding to microtubules. We identify arc-MIPs, a novel class of microtubule inner protein, that bind laterally across protofilaments and remodel tubulin structure and lattice contacts. The binding mechanisms utilized by CA proteins may be generalizable to other microtubule-associated proteins. These structures establish a foundation to elucidate the contributions of individual CA proteins to ciliary motility and ciliopathies.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures














References
-
- Mitchell DR Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil. Cytoskeleton 55, 188–199 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

