Major Features of the 2021 WHO Classification of CNS Tumors
- PMID: 35578106
- PMCID: PMC9723092
- DOI: 10.1007/s13311-022-01249-0
Major Features of the 2021 WHO Classification of CNS Tumors
Abstract
Advances in the understanding of the molecular biology of central nervous system (CNS) tumors prompted a new World Health Organization (WHO) classification scheme in 2021, only 5 years after the prior iteration. The 2016 version was the first to include specific molecular alterations in the diagnoses of a few tumors, but the 2021 system greatly expanded this approach, with over 40 tumor types and subtypes now being defined by their key molecular features. Many tumors have also been reconceptualized into new "supercategories," including adult-type diffuse gliomas, pediatric-type diffuse low- and high-grade gliomas, and circumscribed astrocytic gliomas. Some entirely new tumors are in this scheme, particularly pediatric tumors. Naturally, these changes will impact how CNS tumor patients are diagnosed and treated, including clinical trial enrollment. This review addresses the most clinically relevant changes in the 2021 WHO book, including diffuse and circumscribed gliomas, ependymomas, embryonal tumors, and meningiomas.
Keywords: Astrocytoma; Embryonal; Ependymoma; Glioma; Meningioma; WHO.
© 2022. The American Society for Experimental NeuroTherapeutics, Inc.
Figures
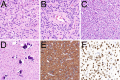

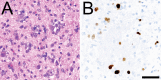

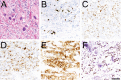
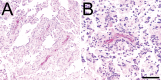
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

