Integrative analysis of scRNA-seq and scATAC-seq revealed transit-amplifying thymic epithelial cells expressing autoimmune regulator
- PMID: 35578835
- PMCID: PMC9113748
- DOI: 10.7554/eLife.73998
Integrative analysis of scRNA-seq and scATAC-seq revealed transit-amplifying thymic epithelial cells expressing autoimmune regulator
Abstract
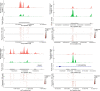
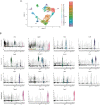
Medullary thymic epithelial cells (mTECs) are critical for self-tolerance induction in T cells via promiscuous expression of tissue-specific antigens (TSAs), which are controlled by the transcriptional regulator, AIRE. Whereas AIRE-expressing (Aire+) mTECs undergo constant turnover in the adult thymus, mechanisms underlying differentiation of postnatal mTECs remain to be discovered. Integrative analysis of single-cell assays for transposase-accessible chromatin (scATAC-seq) and single-cell RNA sequencing (scRNA-seq) suggested the presence of proliferating mTECs with a specific chromatin structure, which express high levels of Aire and co-stimulatory molecules, CD80 (Aire+CD80hi). Proliferating Aire+CD80hi mTECs detected using Fucci technology express a minimal number of Aire-dependent TSAs and are converted into quiescent Aire+CD80hi mTECs expressing high levels of TSAs after a transit amplification. These data provide evidence for the existence of transit-amplifying Aire+mTEC precursors during the Aire+mTEC differentiation process of the postnatal thymus.
Keywords: AIRE; ATAC; differentiation; immunology; inflammation; mouse; single-cell analysis; thymic epithelial cells; transit-amplifying cells.
© 2022, MIyao et al.
Conflict of interest statement
TM, MM, SK, TT, TI, EO, SH, KH, YT, HO, MH, YM, TS, HI, HY, MY, AI, AS, AM, MM, AM, NA, TA No competing interests declared
Figures




























References
-
- Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J-I. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. - DOI - PubMed
-
- Akiyama N, Shinzawa M, Miyauchi M, Yanai H, Tateishi R, Shimo Y, Ohshima D, Matsuo K, Sasaki I, Hoshino K, Wu G, Yagi S, Inoue J, Kaisho T, Akiyama T. Limitation of immune tolerance-inducing thymic epithelial cell development by Spi-B-mediated negative feedback regulation. The Journal of Experimental Medicine. 2014;211:2425–2438. doi: 10.1084/jem.20141207. - DOI - PMC - PubMed
-
- Akiyama N, Takizawa N, Miyauchi M, Yanai H, Tateishi R, Shinzawa M, Yoshinaga R, Kurihara M, Demizu Y, Yasuda H, Yagi S, Wu G, Matsumoto M, Sakamoto R, Yoshida N, Penninger JM, Kobayashi Y, Inoue J-I, Akiyama T. Identification of embryonic precursor cells that differentiate into thymic epithelial cells expressing autoimmune regulator. The Journal of Experimental Medicine. 2016;213:1441–1458. doi: 10.1084/jem.20151780. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

