Multi-tract multi-symptom relationships in pediatric concussion
- PMID: 35579325
- PMCID: PMC9132577
- DOI: 10.7554/eLife.70450
Multi-tract multi-symptom relationships in pediatric concussion
Abstract
Background: The heterogeneity of white matter damage and symptoms in concussion has been identified as a major obstacle to therapeutic innovation. In contrast, most diffusion MRI (dMRI) studies on concussion have traditionally relied on group-comparison approaches that average out heterogeneity. To leverage, rather than average out, concussion heterogeneity, we combined dMRI and multivariate statistics to characterize multi-tract multi-symptom relationships.
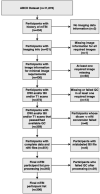
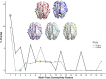
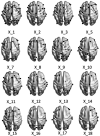
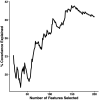
Methods: Using cross-sectional data from 306 previously concussed children aged 9-10 from the Adolescent Brain Cognitive Development Study, we built connectomes weighted by classical and emerging diffusion measures. These measures were combined into two informative indices, the first representing microstructural complexity, the second representing axonal density. We deployed pattern-learning algorithms to jointly decompose these connectivity features and 19 symptom measures.
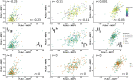
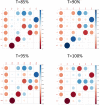
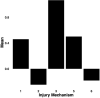
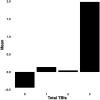
Results: Early multi-tract multi-symptom pairs explained the most covariance and represented broad symptom categories, such as a general problems pair, or a pair representing all cognitive symptoms, and implicated more distributed networks of white matter tracts. Further pairs represented more specific symptom combinations, such as a pair representing attention problems exclusively, and were associated with more localized white matter abnormalities. Symptom representation was not systematically related to tract representation across pairs. Sleep problems were implicated across most pairs, but were related to different connections across these pairs. Expression of multi-tract features was not driven by sociodemographic and injury-related variables, as well as by clinical subgroups defined by the presence of ADHD. Analyses performed on a replication dataset showed consistent results.
Conclusions: Using a double-multivariate approach, we identified clinically-informative, cross-demographic multi-tract multi-symptom relationships. These results suggest that rather than clear one-to-one symptom-connectivity disturbances, concussions may be characterized by subtypes of symptom/connectivity relationships. The symptom/connectivity relationships identified in multi-tract multi-symptom pairs were not apparent in single-tract/single-symptom analyses. Future studies aiming to better understand connectivity/symptom relationships should take into account multi-tract multi-symptom heterogeneity.
Funding: Financial support for this work came from a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research (G.I.G.), an Ontario Graduate Scholarship (S.S.), a Restracomp Research Fellowship provided by the Hospital for Sick Children (S.S.), an Institutional Research Chair in Neuroinformatics (M.D.), as well as a Natural Sciences and Engineering Research Council CREATE grant (M.D.).
Keywords: diffusion MRI; human; medicine; multivariate statistics; neuroscience; pediatric concussions.
Plain language summary
Concussions can damage networks of connections in the brain. Scientists have spent decades and millions of dollars studying concussions and potential treatments. Yet, no new treatments are available or in the pipeline. A major reason for this stagnation is that no two concussions are exactly alike. People affected by concussions may have different genetic or socioeconomic backgrounds. The nature of the injury or how its effects change over time may also vary among people with concussions. One central question facing scientists is whether there are multiple types of concussions. If so, what distinguishes them and what characteristics do they share. Some studies have looked at differences among subgroups of patients with concussions. But questions remain about whether – beyond differences between the patients – the brain injury itself differs and what impact that has on symptoms or patient trajectory. To better characterize different types of concussion, Guberman et al. analyzed diffusion magnetic resonance imaging scans from 306 nine or ten-year-old children with a previous concussion. The children were participants in the Adolescent Brain Cognitive Development Study. Using specialized statistical techniques, the researchers outlined subgroups of concussions in terms of connections and symptoms and studied how many of these subgroups each patient had. Some types of injury were linked with a category of symptoms like cognitive, mood, or physical symptoms. Some types of damage were linked with specific symptoms. Guberman et al. also found that one symptom, sleep problems, was part of many different injury subtypes. Sleep problems may occur in different patients for different reasons. For example, one patient with sleep difficulties may have experienced damage in brain regions controlling sleep and wakefulness. Another person with sleep problems may have injured parts of the brain responsible for mood and may have depression, which causes excessive sleepiness and difficulties waking up. Guberman et al. suggest a new way of thinking about concussions. If more studies confirm these concussion subgroups, scientists might use them to explore which types of therapies might be beneficial for patients with specific subgroups. Developing subgroup-targeted treatments may help scientists overcome the challenges of trying to develop therapies that work across a range of injuries. Similar disease subgrouping strategies may also help researchers study other brain diseases that may vary from patient to patient.
© 2022, Guberman et al.
Conflict of interest statement
GG, SS, EN, AP, DB, AW No competing interests declared, MD works as Chief Scientific Officer for IMEKA. He holds the following patents: DETERMINATION OF WHITE-MATTER NEURODEGENERATIVE DISEASE BIOMARKERS (Patent Application No.: 63/222,914), PROCESSING OF TRACTOGRAPHY RESULTS USING AN AUTOENCODER (Patent Application No.: 17/337,413)
Figures











References
-
- Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Computational Statistics & Data Analysis. 2014;72:176–189. doi: 10.1016/j.csda.2013.11.006. - DOI
-
- Bodien YG, McCrea M, Dikmen S, Temkin N, Boase K, Machamer J, Taylor SR, Sherer M, Levin H, Kramer JH, Corrigan JD, McAllister TW, Whyte J, Manley GT, Giacino JT, TRACK-TBI Investigators Optimizing Outcome Assessment in Multicenter TBI Trials: Perspectives From TRACK-TBI and the TBI Endpoints Development Initiative. The Journal of Head Trauma Rehabilitation. 2018;33:147–157. doi: 10.1097/HTR.0000000000000367. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- U24 DA041147/DA/NIDA NIH HHS/United States
- U01 DA051039/DA/NIDA NIH HHS/United States
- U01 DA041120/DA/NIDA NIH HHS/United States
- U01 DA051018/DA/NIDA NIH HHS/United States
- U01 DA041093/DA/NIDA NIH HHS/United States
- U24 DA041123/DA/NIDA NIH HHS/United States
- U01 DA051038/DA/NIDA NIH HHS/United States
- U01 DA051037/DA/NIDA NIH HHS/United States
- U01 DA051016/DA/NIDA NIH HHS/United States
- U01 DA041106/DA/NIDA NIH HHS/United States
- U01 DA041117/DA/NIDA NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- U01 DA041174/DA/NIDA NIH HHS/United States
- U01 DA041134/DA/NIDA NIH HHS/United States
- U01 DA041022/DA/NIDA NIH HHS/United States
- U01 DA041156/DA/NIDA NIH HHS/United States
- U01 DA050987/DA/NIDA NIH HHS/United States
- U01 DA041025/DA/NIDA NIH HHS/United States
- U01 DA050989/DA/NIDA NIH HHS/United States
- U01 DA041089/DA/NIDA NIH HHS/United States
- U01 DA050988/DA/NIDA NIH HHS/United States
- U01 DA041028/DA/NIDA NIH HHS/United States
- U01 DA041048/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

