The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants
- PMID: 35579533
- PMCID: PMC9161374
- DOI: 10.1126/scitranslmed.abm7621
The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants
Abstract
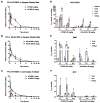
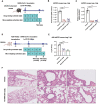
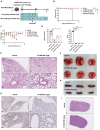
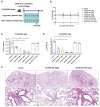
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus driving the ongoing coronavirus disease 2019 (COVID-19) pandemic, continues to rapidly evolve. Because of the limited efficacy of vaccination in prevention of SARS-CoV-2 transmission and continuous emergence of variants of concern (VOCs), orally bioavailable and broadly efficacious antiviral drugs are urgently needed. Previously, we showed that the parent nucleoside of remdesivir, GS-441524, has potent anti-SARS-CoV-2 activity. Here, we report that esterification of the 5'-hydroxyl moieties of GS-441524 markedly improved antiviral potency. This 5'-hydroxyl-isobutyryl prodrug, ATV006, demonstrated excellent oral bioavailability in rats and cynomolgus monkeys and exhibited potent antiviral efficacy against different SARS-CoV-2 VOCs in vitro and in three mouse models. Oral administration of ATV006 reduced viral loads and alleviated lung damage when administered prophylactically and therapeutically to K18-hACE2 mice challenged with the Delta variant of SARS-CoV-2. These data indicate that ATV006 represents a promising oral antiviral drug candidate for SARS-CoV-2.
Figures


References
-
- World Health Organization (WHO), (2022), WHO Coronavirus (COVID-19) Dashboard. Retrieved on May 3,2022 from https://covid19.who.int.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

