Characterization of the SARS-CoV-2 B.1.621 (Mu) variant
- PMID: 35579540
- PMCID: PMC9392899
- DOI: 10.1126/scitranslmed.abm4908
Characterization of the SARS-CoV-2 B.1.621 (Mu) variant
Abstract
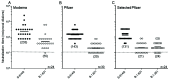
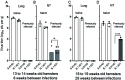
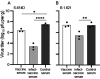
The SARS-CoV-2 B.1.621 (Mu) variant emerged in January 2021 and was categorized as a variant of interest by the World Health Organization in August 2021. This designation prompted us to study the sensitivity of this variant to antibody neutralization. In a live virus neutralization assay with serum samples from individuals vaccinated with the Pfizer/BioNTech or Moderna mRNA vaccines, we measured neutralization antibody titers against B.1.621, an early isolate (spike 614D), and a variant of concern (B.1.351, Beta variant). We observed reduced neutralizing antibody titers against the B.1.621 variant (3.4- to 7-fold reduction, depending on the serum sample and time after the second vaccination) compared to the early isolate and a similar reduction when compared to B.1.351. Likewise, convalescent serum from hamsters previously infected with an early isolate neutralized B.1.621 to a lower degree. Despite this antibody titer reduction, hamsters could not be efficiently rechallenged with the B.1.621 variant, suggesting that the immune response to the first infection is adequate to provide protection against a subsequent infection with the B.1.621 variant.
Figures
References
-
- Laiton-Donato K., Franco-Muñoz C., Álvarez-Díaz D. A., Ruiz-Moreno H. A., Usme-Ciro J. A., Prada D. A., Reales-González J., Corchuelo S., Herrera-Sepúlveda M. T., Naizaque J., Santamaría G., Rivera J., Rojas P., Ortiz J. H., Cardona A., Malo D., Prieto-Alvarado F., Gómez F. R., Wiesner M., Martínez M. L. O., Mercado-Reyes M., Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect. Genet. Evol. 95, 105038 (2021). 10.1016/j.meegid.2021.105038 - DOI - PMC - PubMed
-
- Korber B., Fischer W. M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E. E., Bhattacharya T., Foley B., Hastie K. M., Parker M. D., Partridge D. G., Evans C. M., Freeman T. M., de Silva T. I., McDanal C., Perez L. G., Tang H., Moon-Walker A., Whelan S. P., LaBranche C. C., Saphire E. O., Montefiori D. C.; Sheffield COVID-19 Genomics Group , Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 182, 812–827.e19 (2020). 10.1016/j.cell.2020.06.043 - DOI - PMC - PubMed
-
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M., Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 117, 7001–7003 (2020). 10.1073/pnas.2002589117 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

