Evidence of traumatic brain injury in headbutting bovids
- PMID: 35579705
- PMCID: PMC9217783
- DOI: 10.1007/s00401-022-02427-2
Evidence of traumatic brain injury in headbutting bovids
Abstract
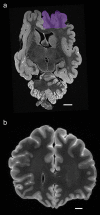
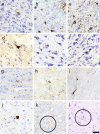
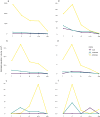
Traumatic brain injury (TBI) is a leading cause of neurologic impairment and death that remains poorly understood. Rodent models have yet to produce clinical therapies, and the exploration of larger and more diverse models remains relatively scarce. We investigated the potential for brain injury after headbutting in two combative bovid species by assessing neuromorphology and neuropathology through immunohistochemistry and stereological quantification. Postmortem brains of muskoxen (Ovibos moschatus, n = 3) and bighorn sheep (Ovis canadensis, n = 4) were analyzed by high-resolution MRI and processed histologically for evidence of TBI. Exploratory histological protocols investigated potential abnormalities in neurons, microglia, and astrocytes in the prefrontal and parietal cortex. Phosphorylated tau protein, a TBI biomarker found in the cerebrospinal fluid and in neurodegenerative lesions, was used to detect possible cellular consequences of chronic or acute TBI. MRI revealed no abnormal neuropathological changes; however, high amounts of tau-immunoreactive neuritic thread clusters, neurites, and neurons were concentrated in the superficial layers of the neocortex, preferentially at the bottom of the sulci in the muskoxen and occasionally around blood vessels. Tau-immunoreactive lesions were rare in the bighorn sheep. Additionally, microglia and astrocytes showed no grouping around tau-immunoreactive cells in either species. Our preliminary findings indicate that muskoxen and possibly other headbutting bovids suffer from chronic or acute brain trauma and that the males' thicker skulls may protect them to a certain extent.
Keywords: CTE; Chronic traumatic encephalopathy; Concussion; MRI; TBI; Tau protein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Centers for Disease Control and Prevention (2019) Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014., ed. U.S. Department of Health and Human Services
-
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee H-S, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. - DOI - PMC - PubMed
-
- Bieniek KF, Cairns NJ, Crary JF, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl DP, Stein TD, Vonsattel J-P. The second NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2021;80(3):210–219. doi: 10.1093/jnen/nlab001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

