Clinical and humanistic burden among pediatric patients with neurofibromatosis type 1 and plexiform neurofibroma in the USA
- PMID: 35579709
- PMCID: PMC9325812
- DOI: 10.1007/s00381-022-05513-8
Clinical and humanistic burden among pediatric patients with neurofibromatosis type 1 and plexiform neurofibroma in the USA
Abstract
Purpose: To assess clinical and humanistic burden among pediatric patients with neurofibromatosis type 1 (NF1) and plexiform neurofibroma (PN) in the USA.
Methods: NF1-PN patients aged 8-18 years (treatment-naïve or ≤ 1 month of selumetinib treatment) and their caregivers and caregivers of similar patients aged 2-7 years were recruited through the Children's Tumor Foundation to participate in an online cross-sectional survey (December 2020-January 2021). Caregivers provided data on patients' demographic and clinical characteristics and burden of debulking surgeries. Patients and caregivers provided self-reported or proxy responses to health-related quality of life (HRQoL) questions using validated instruments.
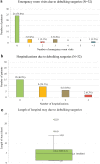
Results: Sixty-one patients and 82 caregivers responded to the survey. Median (range) age of patients was 11.5 (3-18) years, and 53.7% were female. Most were treatment-naïve (97.6%), with NF1-PN diagnosis for > 5 years (68.3%). Most patients (59.8%) had > 1 PN and 11.0% reporting > 5 PNs. Common NF1-PN symptoms included pain (64.6%), disfigurement (32.9%), and motor dysfunction (28.0%). Patients and caregiver proxies reported low overall HRQoL and reduced physical, emotional, social, and school functioning. Patients also reported considerable pain severity, interference, daily activity impairments, and movement difficulty. Few patients had received complete resections of their tumors (12.2%). 39.0% reported ≥ 1 debulking surgery, among whom, 15.6% had complications, and debulking surgery-related hospitalizations were common (53.1%).
Conclusions: The clinical and humanistic burden among pediatric NF1-PN patients is substantial. While debulking surgeries are used for symptom management, they are associated with considerable clinical sequelae. Results highlight a need for improved disease management strategies.
Keywords: Debulking surgery; Health-related quality of life; Neurofibromatosis type 1; Patient-reported outcomes; Plexiform neurofibroma.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
XY is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and owns stock in Merck & Co., Inc., Kenilworth, NJ, USA. HKY is an employee of AstraZeneca and owns stock. SA was an employee of AstraZeneca and owned stock during the conduct of the study. WYC, SS, LZ, and MSD are employees of Analysis Group, Inc., which received funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, in collaboration with AstraZeneca UK Limited for the conduct of the present study.
Figures
References
-
- Friedman J (2019) Neurofibromatosis 1. GeneReviews®[Internet]
-
- Foundation CsT (2021) Diagnostic criteria for neurofibromatosis type 1 (NF1). In: Foundation CsT, editor
-
- National Cancer Institute: Neurofibromatosis type 1 and cancer susceptibility (2021). https://dceg.cancer.gov/research/what-we-study/neurofibromatosis-cancer-.... Accessed 22 Apr 2021
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

