The reproductive effects of the cancer chemotherapy agent, Carmofur, on Daphnia magna are mediated by its metabolite, 5-Fluorouracil
- PMID: 35579761
- PMCID: PMC9233140
- DOI: 10.1007/s10646-022-02551-5
The reproductive effects of the cancer chemotherapy agent, Carmofur, on Daphnia magna are mediated by its metabolite, 5-Fluorouracil
Abstract
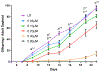
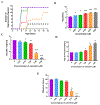
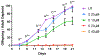
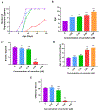
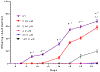
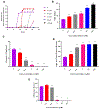
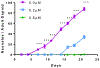
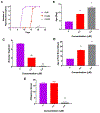
Carmofur is an antineoplastic agent that inhibits ceramidase, a key enzyme in the sphingolipid pathway. Previous research suggests carmofur represses reproductive maturity in Daphnia magna. The purpose of this experiment was to confirm carmofur's effects on fecundity and reproductive maturity over two generations. A chronic toxicity test found reproductive maturity was delayed from 9 to 19 days by 0.80 μM carmofur with a 99.7% drop in reproduction, probably caused by delayed ovarian development. Second generation effects were even greater with 0% reproductive success at 0.40 μM. To our surprise, carmofur was not measured in the media by HPLC 24 h after exposure. Previous research indicated that carmofur is unstable in water and hydrolyzed into 5-fluorouracil (5-FU). Therefore, the chronic toxicity study was repeated with 5-FU and similar effects on reproductive maturity were observed at similar concentrations despite very different acute toxicities (48 h carmofur LC50 = 1.93 μM; 5-FU LC50 = 207 μM). 5-FU delayed reproductive maturity from 9 to 21 days with a 71.12% drop in reproduction at 0.80 μM and greater effects in the 2nd generation similar to carmofur. 5-FU was found stable in aquatic media and HPLC confirmed 5-FU was hydrolyzed from carmofur within 24 h. In conclusion, carmofur and 5-FU reduce fecundity because they delay reproductive maturity and ovarian development in Daphnia magna. We conclude that the reproductive effects observed after carmofur treatment are primarily mediated by its breakdown product, 5-FU. This further underscores the importance of measuring chemical concentrations and evaluating chemical metabolism and decomposition when determining toxicity, especially of chemotherapeutic agents.Clinical trials registration Not applicable.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of Interest
The authors have no conflicts of interest to declare
Figures

References
-
- Baldwin WS, Bailey R, Long KE, Klaine S (2001). Incomplete ecdysis is an indicator of ecdysteroid exposure in Daphnia magna. Environ Toxicol Chem 20:1564–1569. - PubMed
-
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi J-H, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak R, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Zdobnov EM, Grigoriev IV,Lynch M, Boore JL (2011) The ecoresponsive genome of Daphnia pulex. Science 331:555–561. - PMC - PubMed
-
- Fox DR (2010). A Bayesian approach for determining the no effect concentration and hazardous concentration in ecotoxicology. Ectoxicol Environ Saf 73:123–131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

