Stromal Notch ligands foster lymphopenia-driven functional plasticity and homeostatic proliferation of naive B cells
- PMID: 35579963
- PMCID: PMC9246379
- DOI: 10.1172/JCI158885
Stromal Notch ligands foster lymphopenia-driven functional plasticity and homeostatic proliferation of naive B cells
Abstract
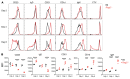
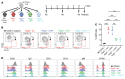
In lymphopenic environments, secondary lymphoid organs regulate the size of B and T cell compartments by supporting the homeostatic proliferation of mature lymphocytes. The molecular mechanisms underlying these responses and their functional consequences remain incompletely understood. To evaluate homeostasis of the mature B cell pool during lymphopenia, we turned to an adoptive transfer model of purified follicular B cells into Rag2-/- mouse recipients. Highly purified follicular B cells transdifferentiated into marginal zone-like B cells when transferred into Rag2-/- lymphopenic hosts but not into wild-type hosts. In lymphopenic spleens, transferred B cells gradually lost their follicular phenotype and acquired characteristics of marginal zone B cells, as judged by cell surface phenotype, expression of integrins and chemokine receptors, positioning close to the marginal sinus, and an ability to rapidly generate functional plasma cells. Initiation of follicular to marginal zone B cell transdifferentiation preceded proliferation. Furthermore, the transdifferentiation process was dependent on Notch2 receptors in B cells and expression of Delta-like 1 Notch ligands by splenic Ccl19-Cre+ fibroblastic stromal cells. Gene expression analysis showed rapid induction of Notch-regulated transcripts followed by upregulated Myc expression and acquisition of broad transcriptional features of marginal zone B cells. Thus, naive mature B cells are endowed with plastic transdifferentiation potential in response to increased stromal Notch ligand availability during lymphopenia.
Keywords: Adaptive immunity; Immunoglobulins; Immunology.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

