Systematic Evidence Map for Over One Hundred and Fifty Per- and Polyfluoroalkyl Substances (PFAS)
- PMID: 35580034
- PMCID: PMC9113544
- DOI: 10.1289/EHP10343
Systematic Evidence Map for Over One Hundred and Fifty Per- and Polyfluoroalkyl Substances (PFAS)
Erratum in
-
Erratum: "Systematic Evidence Map for over One Hundred and Fifty Per- and Polyfluoroalkyl Substances (PFAS)".Environ Health Perspect. 2024 Jan;132(1):19001. doi: 10.1289/EHP14191. Epub 2024 Jan 10. Environ Health Perspect. 2024. PMID: 38198380 Free PMC article. No abstract available.
Abstract
Background: Per- and polyfluoroalkyl substances (PFAS) are a large class of synthetic (man-made) chemicals widely used in consumer products and industrial processes. Thousands of distinct PFAS exist in commerce. The 2019 U.S. Environmental Protection Agency (U.S. EPA) Per- and Polyfluoroalkyl Substances (PFAS) Action Plan outlines a multiprogram national research plan to address the challenge of PFAS. One component of this strategy involves the use of systematic evidence map (SEM) approaches to characterize the evidence base for hundreds of PFAS.
Objective: SEM methods were used to summarize available epidemiological and animal bioassay evidence for a set of PFAS that were prioritized in 2019 by the U.S. EPA's Center for Computational Toxicology and Exposure (CCTE) for in vitro toxicity and toxicokinetic assay testing.
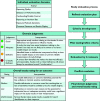
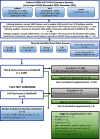
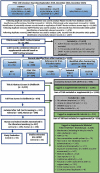
Methods: Systematic review methods were used to identify and screen literature using manual review and machine-learning software. The Populations, Exposures, Comparators, and Outcomes (PECO) criteria were kept broad to identify mammalian animal bioassay and epidemiological studies that could inform human hazard identification. A variety of supplemental content was also tracked, including information on in vitro model systems; exposure measurement-only studies in humans; and absorption, distribution, metabolism, and excretion (ADME). Animal bioassay and epidemiology studies meeting PECO criteria were summarized with respect to study design, and health system(s) were assessed. Because animal bioassay studies with exposure duration (or reproductive/developmental study design) were most useful to CCTE analyses, these studies underwent study evaluation and detailed data extraction. All data extraction is publicly available online as interactive visuals with downloadable metadata.
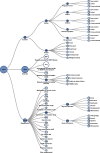
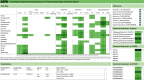
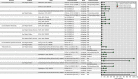
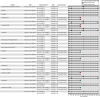
Results: More than 40,000 studies were identified from scientific databases. Screening processes identified 44 animal and 148 epidemiology studies from the peer-reviewed literature and 95 animal and 50 epidemiology studies from gray literature that met PECO criteria. Epidemiological evidence (available for 15 PFAS) mostly assessed the reproductive, endocrine, developmental, metabolic, cardiovascular, and immune systems. Animal evidence (available for 40 PFAS) commonly assessed effects in the reproductive, developmental, urinary, immunological, and hepatic systems. Overall, 45 PFAS had evidence across animal and epidemiology data streams.
Discussion: Many of the PFAS were data poor. Epidemiological and animal evidence were lacking for most of the PFAS included in our search. By disseminating this information, we hope to facilitate additional assessment work by providing the initial scoping literature survey and identifying key research needs. Future research on data-poor PFAS will help support a more complete understanding of the potential health effects from PFAS exposures. https://doi.org/10.1289/EHP10343.
Figures



Comment in
-
Invited Perspective: The Promise of Fit-for-Purpose Systematic Evidence Maps for Supporting Regulatory Health Assessment.Environ Health Perspect. 2022 May;130(5):51303. doi: 10.1289/EHP10743. Epub 2022 May 17. Environ Health Perspect. 2022. PMID: 35580037 Free PMC article. No abstract available.
References
-
- Office of Economic Cooperation and Development. 2018. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs). ENV/JM/MONO(2018)7. http://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/ [accessed 4 January 2021].
-
- Office of Economic Cooperation and Development. 2021. Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance. https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/term... [accessed 7 December 2021].
-
- Interstate Technology and Regulatory Council. 2021. PFAS Technical and Regulatory Guidance Document. https://pfas-1.itrcweb.org/ [accessed 7 December 2021].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
