Maternal undernutrition during periconceptional period affects whole-genome ovine muscle methylation in adult offspring
- PMID: 35580043
- PMCID: PMC9387600
- DOI: 10.1093/jas/skac180
Maternal undernutrition during periconceptional period affects whole-genome ovine muscle methylation in adult offspring
Abstract
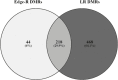
Experimental and epidemiological studies suggest that maternal nutritional status during early pregnancy, including the period around the time of conception, may induce long-lasting epigenetic changes in the offspring. However, this remains largely unexplored in livestock. Therefore, the objective of this study was to evaluate if modification of the maternal diet of sheep (CTR: control; UND: 50% undernutrition) during the periconceptional period (42 d in total: -14/+28 from mating), would impact CpG methylation in muscle tissue (Longissimus dorsi) of adult offspring (11.5 mo old). Reduced representation bisulfite sequencing identified 262 (Edge-R, FDR < 0.05) and 686 (logistic regression, FDR < 0.001) differentially methylated regions (DMRs) between the UND and CTR groups. Gene ontology analysis identified genes related to development, functions of the muscular system, and steroid hormone receptor activity within the DMRs. The data reported here show that nutritional stress during early pregnancy leads to epigenetic modifications in the muscle of the resulting offspring, with possible implications for cardiac dysfunction, muscle physiology, and meat production.
Keywords: diet; epigenetics; methylation; muscle; undernutrition.
Plain language summary
The formation of the epigenetic pattern of an organism is highly sensitive to environmental factors, especially during early mammalian development, when epigenetic reprogramming of the whole genome takes place. In utero adverse conditions experienced during early pregnancy, such as maternal undernutrition, may induce long-lasting epigenetic changes in the resulting offspring. This study investigated the CpG methylation variations in muscle tissue of adult offspring induced by differences in the diet of their mothers during pregnancy. Our data show that undernutrition during pregnancy leads to epigenetic alterations in the muscle of the offspring, with a potential impact on animal health and productivity.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Maternal peri-conceptional undernourishment perturbs offspring sperm methylome.Reproduction. 2020 May;159(5):513-523. doi: 10.1530/REP-19-0549. Reproduction. 2020. PMID: 32103819
-
Integrative analysis of methylomic and transcriptomic data in fetal sheep muscle tissues in response to maternal diet during pregnancy.BMC Genomics. 2018 Feb 6;19(1):123. doi: 10.1186/s12864-018-4509-0. BMC Genomics. 2018. PMID: 29409445 Free PMC article.
-
Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring.Endocrinology. 2013 Dec;154(12):4560-9. doi: 10.1210/en.2013-1693. Epub 2013 Sep 24. Endocrinology. 2013. PMID: 24064364
-
Evidence for similar changes in offspring phenotype following either maternal undernutrition or overnutrition: potential impact on fetal epigenetic mechanisms.Reprod Fertil Dev. 2011;24(1):105-11. doi: 10.1071/RD11911. Reprod Fertil Dev. 2011. PMID: 22394722 Review.
-
Maternal nutritional status, C(1) metabolism and offspring DNA methylation: a review of current evidence in human subjects.Proc Nutr Soc. 2012 Feb;71(1):154-65. doi: 10.1017/S0029665111003338. Epub 2011 Nov 29. Proc Nutr Soc. 2012. PMID: 22124338 Free PMC article. Review.
Cited by
-
Effect of maternal periconceptional undernutrition in sheep on cortisol regulation in offspring from mid-late gestation, through to adulthood.Front Endocrinol (Lausanne). 2023 Feb 2;14:1122432. doi: 10.3389/fendo.2023.1122432. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36817600 Free PMC article.
References
-
- Bindea, G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A., Fridman W. H., Pagès F., Trajanoski Z., and Galon J... 2009. ClueGO, a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 25:1091–1093. doi:10.1093/bioinformatics/btp101 - DOI - PMC - PubMed
-
- Boyer, J. G., Prasad V., Song T., Lee D., Fu X., Grimes K. M., Sargent M. A., Sadayappan S., and Molkentin J. D... 2019. ERK1/2 signaling induces skeletal muscle slow fiber-type switching and reduces muscular dystrophy disease severity. JCI Insight. 5:e127356. doi:10.1172/jci.insight.127356 - DOI - PMC - PubMed