Oxidative Fluorination of Heteroatoms Enabled by Trichloroisocyanuric Acid and Potassium Fluoride
- PMID: 35580251
- PMCID: PMC9400999
- DOI: 10.1002/anie.202205088
Oxidative Fluorination of Heteroatoms Enabled by Trichloroisocyanuric Acid and Potassium Fluoride
Abstract
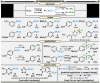
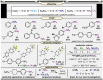
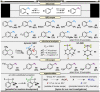
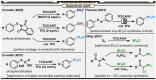
In synthetic method development, the most rewarding path is seldom a straight line. While our initial entry into pentafluorosulfanyl (SF5 ) chemistry did not go according to plan (due to inaccessibility of reagents such as SF5 Cl at the time), a "detour" led us to establish mild and inexpensive oxidative fluorination conditions that made aryl-SF5 compound synthesis more accessible. The method involved the use of potassium fluoride and trichloroisocyanuric acid (TCICA)-a common swimming pool disinfectant-as opposed to previously employed reagents such as F2 , XeF2 , HF, and Cl2 . Thereafter, curiosity led us to explore applications of TCICA/KF as a more general approach to the synthesis of fluorinated Group 15, 16, and 17 heteroatoms in organic scaffolds; this, in turn, prompted SC-XRD, VT-NMR, computational, and physical organic studies. Ultimately, it was discovered that TCICA/KF can be used to synthesize SF5 Cl, enabling SF5 chemistry in an unexpected way.
Keywords: Gas-Reagent-Free; Inorganic Fluorine Chemistry; Oxidative Fluorination; Pentafluorosulfanyl; Trichloroisocyanuric Acid.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

