Safety and efficacy of a dose-dense short-term therapy in patients with MYC-translocated aggressive lymphoma
- PMID: 35580327
- PMCID: PMC9641166
- DOI: 10.1182/bloodadvances.2022007475
Safety and efficacy of a dose-dense short-term therapy in patients with MYC-translocated aggressive lymphoma
Abstract
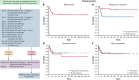
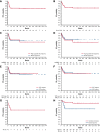
Patients with aggressive B-cell lymphoma and MYC rearrangement at fluorescence in situ hybridization exhibit poor outcome after R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). In the last decade, 68 patients with Burkitt lymphoma ([BL] n = 46) or high-grade B-cell lymphoma ([HGBCL] single, double, or triple hit; n = 22) were treated with a dose-dense, short-term therapy termed "CARMEN regimen" at 5 Italian centers. Forty-six (68%) patients were HIV+. CARMEN included a 36-day induction with sequential, single weekly doses of cyclophosphamide, vincristine, rituximab, methotrexate, etoposide, and doxorubicin plus intrathecal chemotherapy, followed by high-dose-cytarabine-based consolidation. Patients who did not achieve complete remission (CR) after induction received BEAM (carmustina, etoposide, cytarabine, melfalan)-conditioned autologous stem cell transplantation (ASCT) after consolidation. Sixty-one (90%) patients completed induction, and 59 (87%) completed consolidation. Seventeen patients received ASCT. Grade 4 hematological toxicity was common but did not cause treatment discontinuation; grade 4 nonhematological toxicity was recorded in 11 (16%) patients, with grade 4 infections in 6 (9%). Six (9%) patients died of toxicity (sepsis in 4, COVID-19, acute respiratory distress syndrome). CR rate after the whole treatment was 73% (95% confidence interval [CI], 55% to 91%) for patients with HGBCL and 78% (95% CI, 66% to 90%) for patients with BL. At a median follow-up of 65 (interquartile range, 40-109) months, 48 patients remain event free, with a 5-year progression-free survival of 63% (95% CI, 58% to 68%) for HGBCL and 72% (95% CI, 71% to 73%) for BL, with a 5-year overall survival (OS) of 63% (95% CI, 58% to 68%) and 76% (95% CI, 75% to 77%), respectively. HIV seropositivity did not have a detrimental effect on outcome. This retrospective study shows that CARMEN is a safe and active regimen both in HIV-negative and -positive patients with MYC-rearranged lymphomas. Encouraging survival figures, attained with a single dose of doxorubicin and cyclophosphamide, deserve further investigation in HGBCL and other aggressive lymphomas.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Kluin P.M, Harris NL, Stein H, et al. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, et al., editors. IARC; Lyon, France: 2017. High-grade B-cell lymphoma; pp. 335–341.
-
- Kluin PM, Harris NL, Stein H, et al. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, et al., editors. IARC; Lyon, France: 2008.
-
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. - PubMed
-
- Laude MC, Lebras L, Sesques P, et al. First-line treatment of double-hit and triple-hit lymphomas: Survival and tolerance data from a retrospective multicenter French study. Am J Hematol. 2021;96(3):302–311. - PubMed
-
- Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113(1):117–125. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

