Role of Nitric Oxide-Releasing Glycosaminoglycans in Wound Healing
- PMID: 35580341
- PMCID: PMC11574979
- DOI: 10.1021/acsbiomaterials.2c00392
Role of Nitric Oxide-Releasing Glycosaminoglycans in Wound Healing
Abstract
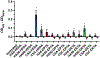
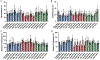
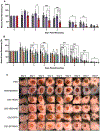
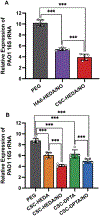
Two glycosaminoglycan (GAG) biopolymers, hyaluronic acid (HA) and chondroitin sulfate (CS), were chemically modified via carbodiimide chemistry to facilitate the loading and release of nitric oxide (NO) to develop a multi-action wound healing agent. The resulting NO-releasing GAGs released 0.2-0.9 μmol NO mg-1 GAG into simulated wound fluid with NO-release half-lives ranging from 20 to 110 min. GAGs containing alkylamines with terminal primary amines and displaying intermediate NO-release kinetics exhibited potent, broad spectrum bactericidal action against three strains each of Pseudomonas aeruginosa and Staphylococcus aureus ranging in antibiotic resistance profile. NO loading of the GAGs was also found to decrease murine TLR4 activation, suggesting that the therapeutic exhibits anti-inflammatory mechanisms. In vitro adhesion and proliferation assays utilizing human dermal fibroblasts and human epidermal keratinocytes displayed differences as a function of the GAG backbone, alkylamine identity, and NO-release properties. In combination with antibacterial properties, the adhesion and proliferation profiles of the GAG derivatives enabled the selection of the most promising wound healing candidates for subsequent in vivo studies. A P. aeruginosa-infected murine wound model revealed the benefits of CS over HA as a pro-wound healing NO donor scaffold, with benefits of accelerated wound closure and decreased bacterial burden attributable to both active NO release and the biopolymer backbone.
Keywords: antibacterial; chondroitin sulfate; hyaluronic acid; nitric oxide; wound healing.
Figures
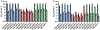
References
-
- Stechmiller JK; Childress B; Cowan L Arginine Supplementation and Wound Healing. 2005, 52–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
