Amyloid β Dodecamer Disrupts the Neuronal Membrane More Strongly than the Mature Fibril: Understanding the Role of Oligomers in Neurotoxicity
- PMID: 35580354
- PMCID: PMC9150093
- DOI: 10.1021/acs.jpcb.2c01769
Amyloid β Dodecamer Disrupts the Neuronal Membrane More Strongly than the Mature Fibril: Understanding the Role of Oligomers in Neurotoxicity
Abstract
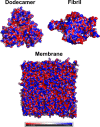
The amyloid cascade hypothesis states that senile plaques, composed of amyloid β (Aβ) fibrils, play a key role in Alzheimer's disease (AD). However, recent experiments have shown that Aβ oligomers are more toxic to neurons than highly ordered fibrils. The molecular mechanism underlying this observation remains largely unknown. One of the possible scenarios for neurotoxicity is that Aβ peptides create pores in the lipid membrane that allow Ca2+ ions to enter cells, resulting in a signal of cell apoptosis. Hence, one might think that oligomers are more toxic due to their higher ability to create ion channels than fibrils. In this work, we study the effect of Aβ42 dodecamer and fibrils on a neuronal membrane, which is similar to that observed in AD patients, using all-atom molecular dynamics simulations. Due to short simulation times, we cannot observe the formation of pores, but useful insight on the early events of this process has been obtained. Namely, we showed that dodecamer distorts the lipid membrane to a greater extent than fibrils, which may indicate that ion channels can be more easily formed in the presence of oligomers. Based on this result, we anticipate that oligomers are more toxic than mature fibrils, as observed experimentally. Moreover, the Aβ-membrane interaction was found to be governed by the repulsive electrostatic interaction between Aβ and the ganglioside GM1 lipid. We calculated the bending and compressibility modulus of the membrane in the absence of Aβ and obtained good agreement with the experiment. We predict that the dodecamer will increase the compressibility modulus but has little effect on the bending modulus. Due to the weak interaction with the membrane, fibrils insignificantly change the membrane elastic properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
How do membranes initiate Alzheimer's Disease? Formation of toxic amyloid fibrils by the amyloid β-protein on ganglioside clusters.Acc Chem Res. 2014 Aug 19;47(8):2397-404. doi: 10.1021/ar500127z. Epub 2014 Jul 16. Acc Chem Res. 2014. PMID: 25029558
-
Monosialotetrahexosylganglioside Promotes Early Aβ42 Oligomer Formation and Maintenance.ACS Chem Neurosci. 2022 Jul 6;13(13):1979-1991. doi: 10.1021/acschemneuro.2c00221. Epub 2022 Jun 17. ACS Chem Neurosci. 2022. PMID: 35713284 Free PMC article.
-
Amyloid-β Peptide Nitrotyrosination Stabilizes Oligomers and Enhances NMDAR-Mediated Toxicity.J Neurosci. 2016 Nov 16;36(46):11693-11703. doi: 10.1523/JNEUROSCI.1081-16.2016. J Neurosci. 2016. PMID: 27852777 Free PMC article.
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Chronic Sleep Deprivation Altered the Expression of Memory-Related Genes and Caused Cognitive Memory Dysfunction in Mice.Int J Mol Sci. 2024 Oct 29;25(21):11634. doi: 10.3390/ijms252111634. Int J Mol Sci. 2024. PMID: 39519186 Free PMC article.
-
Dissociation process of polyalanine aggregates by free electron laser irradiation.PLoS One. 2023 Sep 8;18(9):e0291093. doi: 10.1371/journal.pone.0291093. eCollection 2023. PLoS One. 2023. PMID: 37683014 Free PMC article.
-
Inhibition of amyloid-β(16-22) aggregation by polyphenols using replica permutation with solute tempering molecular dynamics simulation.Biophys Physicobiol. 2023 Dec 9;20(4):e200045. doi: 10.2142/biophysico.bppb-v20.0045. eCollection 2023. Biophys Physicobiol. 2023. PMID: 38344035 Free PMC article.
-
Potential Mechanisms of Tunneling Nanotube Formation and Their Role in Pathology Spread in Alzheimer's Disease and Other Proteinopathies.Int J Mol Sci. 2024 Oct 8;25(19):10797. doi: 10.3390/ijms251910797. Int J Mol Sci. 2024. PMID: 39409126 Free PMC article. Review.
-
Recent Computational Advances Regarding Amyloid-β and Tau Membrane Interactions in Alzheimer's Disease.Molecules. 2023 Oct 13;28(20):7080. doi: 10.3390/molecules28207080. Molecules. 2023. PMID: 37894559 Free PMC article. Review.
References
-
- 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dementia, 2018; Vol. 14, pp 367–429.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

