Convergent evolution of toxin resistance in animals
- PMID: 35580905
- PMCID: PMC9543476
- DOI: 10.1111/brv.12865
Convergent evolution of toxin resistance in animals
Abstract
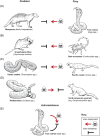
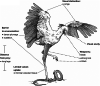
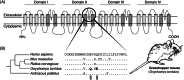
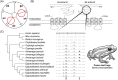
Convergence is the phenomenon whereby similar phenotypes evolve independently in different lineages. One example is resistance to toxins in animals. Toxins have evolved many times throughout the tree of life. They disrupt molecular and physiological pathways in target species, thereby incapacitating prey or deterring a predator. In response, molecular resistance has evolved in many species exposed to toxins to counteract their harmful effects. Here, we review current knowledge on the convergence of toxin resistance using examples from a wide range of toxin families. We explore the evolutionary processes and molecular adaptations driving toxin resistance. However, resistance adaptations may carry a fitness cost if they disrupt the normal physiology of the resistant animal. Therefore, there is a trade-off between maintaining a functional molecular target and reducing toxin susceptibility. There are relatively few solutions that satisfy this trade-off. As a result, we see a small set of molecular adaptations appearing repeatedly in diverse animal lineages, a phenomenon that is consistent with models of deterministic evolution. Convergence may also explain what has been called 'autoresistance'. This is often thought to have evolved for self-protection, but we argue instead that it may be a consequence of poisonous animals feeding on toxic prey. Toxin resistance provides a unique and compelling model system for studying the interplay between trophic interactions, selection pressures and the molecular mechanisms underlying evolutionary novelties.
Keywords: co-evolutionary arms races; convergent evolution; deterministic evolution; functional constraint; molecular adaptation; toxin resistance.
© 2022 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures




References
-
- Abderemane‐Ali, F. , Rossen, N. D. , Kobiela, M. E. , Craig, R. A. II , Garrison, C. E. , Chen, Z. , Colleran, C. M. , O'Connell, L. A. , Du Bois, J. , Dumbacher, J. P. & Minor, D. L. Jr. (2021). Evidence that toxin resistance in poison birds and frogs is not rooted in sodium channel mutations and may rely on “toxin sponge” proteins. Journal of General Physiology 153, 1–20. - PMC - PubMed
-
- Albuquerque, E. X. , Daly, J. W. & Witkop, B. (1971). Batrachotoxin: chemistry and pharmacology. Science 172, 995–1002. - PubMed
-
- Alexander, G. J. & Maritz, B. (2010). Bitis arietans arietans: partial resistance to Naja venom. African Herp News 50, 34–36.
-
- Almeida‐Santos, S. M. , Antoniazzi, M. M. , Sant'Anna, O. A. & Jared, C. (2000). Predation by the opossum Didelphis marsupialis on the rattlesnake Crotalus durissus . Current Herpetology 19, 1–9.
-
- Aráoz, R. , Molgó, J. & Tandeau de Marsac, N. (2010). Neurotoxic cyanobacterial toxins. Toxicon 56, 813–828. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

