Prereferral rectal artesunate and referral completion among children with suspected severe malaria in the Democratic Republic of the Congo, Nigeria and Uganda
- PMID: 35580913
- PMCID: PMC9114942
- DOI: 10.1136/bmjgh-2021-008346
Prereferral rectal artesunate and referral completion among children with suspected severe malaria in the Democratic Republic of the Congo, Nigeria and Uganda
Abstract
Introduction: Children who receive prereferral rectal artesunate (RAS) require urgent referral to a health facility where appropriate treatment for severe malaria can be provided. However, the rapid improvement of a child's condition after RAS administration may influence a caregiver's decision to follow this recommendation. Currently, the evidence on the effect of RAS on referral completion is limited.
Methods: An observational study accompanied the roll-out of RAS in three malaria endemic settings in the Democratic Republic of the Congo (DRC), Nigeria and Uganda. Community health workers and primary health centres enrolled children under 5 years with suspected severe malaria before and after the roll-out of RAS. All children were followed up 28 days after enrolment to assess their treatment-seeking pathways.
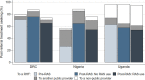
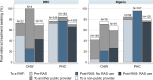
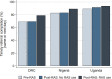
Results: Referral completion was 67% (1408/2104) in DRC, 48% (287/600) in Nigeria and 58% (2170/3745) in Uganda. In DRC and Uganda, RAS users were less likely to complete referral than RAS non-users in the pre-roll-out phase (adjusted OR (aOR)=0.48, 95% CI 0.30 to 0.77 and aOR=0.72, 95% CI 0.58 to 0.88, respectively). Among children seeking care from a primary health centre in Nigeria, RAS users were less likely to complete referral compared with RAS non-users in the post-roll-out phase (aOR=0.18, 95% CI 0.05 to 0.71). In Uganda, among children who completed referral, RAS users were significantly more likely to complete referral on time than RAS non-users enrolled in the pre-roll-out phase (aOR=1.81, 95% CI 1.17 to 2.79).
Conclusions: The findings of this study raise legitimate concerns that the roll-out of RAS may lead to lower referral completion in children who were administered prereferral RAS. To ensure that community-based programmes are effectively implemented, barriers to referral completion need to be addressed at all levels. Alternative effective treatment options should be provided to children unable to complete referral.
Trial registrstion number: NCT03568344; ClinicalTrials.gov.
Keywords: child health; health policy; malaria.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Effectiveness of rectal artesunate as pre-referral treatment for severe malaria in children under 5 years of age: a multi-country observational study.BMC Med. 2022 Oct 11;20(1):343. doi: 10.1186/s12916-022-02541-8. BMC Med. 2022. PMID: 36217159 Free PMC article. Clinical Trial.
-
Health worker compliance with severe malaria treatment guidelines in the context of implementing pre-referral rectal artesunate in the Democratic Republic of the Congo, Nigeria, and Uganda: An operational study.PLoS Med. 2023 Feb 21;20(2):e1004189. doi: 10.1371/journal.pmed.1004189. eCollection 2023 Feb. PLoS Med. 2023. PMID: 36809247 Free PMC article.
-
Acceptability of pre-referral rectal artesunate for severe malaria in children under 5 years by health workers and caregivers in the Democratic Republic of the Congo, Nigeria and Uganda.Malar J. 2022 Nov 10;21(1):322. doi: 10.1186/s12936-022-04348-7. Malar J. 2022. PMID: 36357894 Free PMC article.
-
Pre-referral rectal artesunate: no cure for unhealthy systems.Lancet Infect Dis. 2023 Jun;23(6):e213-e217. doi: 10.1016/S1473-3099(22)00762-9. Epub 2022 Dec 19. Lancet Infect Dis. 2023. PMID: 36549311 Review.
-
The wrongful indictment of pre-referral rectal artesunate further delays the roll-out of this lifesaving drug.Lancet Infect Dis. 2023 Jun;23(6):e208-e212. doi: 10.1016/S1473-3099(22)00765-4. Epub 2022 Dec 19. Lancet Infect Dis. 2023. PMID: 36549312 Review.
Cited by
-
WHO should accelerate, not stall, rectal artesunate deployment for pre-referral treatment of severe malaria.Trans R Soc Trop Med Hyg. 2023 Jul 4;117(7):536-538. doi: 10.1093/trstmh/trad002. Trans R Soc Trop Med Hyg. 2023. PMID: 36722432 Free PMC article.
-
Defining the next generation of severe malaria treatment: a target product profile.Malar J. 2024 Jun 5;23(1):174. doi: 10.1186/s12936-024-04986-z. Malar J. 2024. PMID: 38835069 Free PMC article. Review.
-
Assessing caregivers' perceptions of treatment-seeking for suspected severe malaria in the Democratic Republic of the Congo.Malar J. 2023 Oct 13;22(1):308. doi: 10.1186/s12936-023-04737-6. Malar J. 2023. PMID: 37828524 Free PMC article.
-
Stopping prereferral rectal artesunate - a grave error.BMJ Glob Health. 2022 Jul;7(7):e010006. doi: 10.1136/bmjgh-2022-010006. BMJ Glob Health. 2022. PMID: 35831037 Free PMC article. No abstract available.
-
Child acute illness presentation and referrals at primary health clinics in Malawi: a secondary analysis of ASPIRE.BMJ Open. 2024 Apr 25;14(4):e079589. doi: 10.1136/bmjopen-2023-079589. BMJ Open. 2024. PMID: 38670607 Free PMC article.
References
-
- Global Malaria Programme . Rectal artesunate for pre-referral treatment of severe malaria. Geneva: World Health Organization; 2017.
-
- World Health Organization . Guidelines for the treatment of malaria - 3rd edition. Geneva: World Health Organization; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
