CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy
- PMID: 35580929
- PMCID: PMC9114852
- DOI: 10.1136/jitc-2021-004022
CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy
Erratum in
-
Correction: CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy.J Immunother Cancer. 2022 Oct;10(10):e004022corr1. doi: 10.1136/jitc-2021-004022corr1. J Immunother Cancer. 2022. PMID: 36283738 Free PMC article. No abstract available.
Abstract
Background: Antitumor therapeutic vaccines are generally based on antigenic epitopes presented by major histocompatibility complex (MHC-I) molecules to induce tumor-specific CD8+ T cells. Paradoxically, continuous T cell receptor (TCR) stimulation from tumor-derived CD8+ T-cell epitopes can drive the functional exhaustion of tumor-specific CD8+ T cells. Tumor-specific type-I helper CD4+ T (TH1) cells play an important role in the population maintenance and cytotoxic function of exhausted tumor-specific CD8+ T cells in the tumor microenvironment. Nonetheless, whether the vaccination strategy targeting MHC-II-restricted CD4+ T-cell epitopes to induce tumor-specific TH1 responses can confer effective antitumor immunity to restrain tumor growth is not well studied. Here, we developed a heterologous prime-boost vaccination strategy to effectively induce tumor-specific TH1 cells and evaluated its antitumor efficacy and its capacity to potentiate PD-1/PD-L1 immunotherapy.
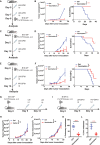
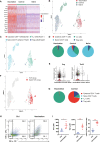
Methods: Listeria monocytogenes vector and influenza A virus (PR8 strain) vector stably expressing lymphocytic choriomeningitis virus (LCMV) glycoprotein-specific I-Ab-restricted CD4+ T cell epitope (GP61-80) or ovalbumin-specific CD4+ T cell epitope (OVA323-339) were constructed and evaluated their efficacy against mouse models of melanoma and colorectal adenocarcinoma expressing lymphocytic choriomeningitis virus glycoprotein and ovalbumin. The impact of CD4+ T cell epitope-based heterologous prime-boost vaccination was detected by flow-cytometer, single-cell RNA sequencing and single-cell TCR sequencing.
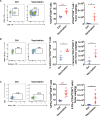
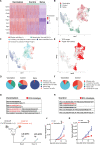
Results: CD4+ T cell epitope-based heterologous prime-boost vaccination efficiently suppressed both mouse melanoma and colorectal adenocarcinoma. This vaccination primarily induced tumor-specific TH1 response, which in turn enhanced the expansion, effector function and clonal breadth of tumor-specific CD8+ T cells. Furthermore, this vaccination strategy synergized PD-L1 blockade mediated tumor suppression. Notably, prime-boost vaccination extended the duration of PD-L1 blockade induced antitumor effects by preventing the re-exhaustion of tumor-specific CD8+ T cells.
Conclusion: CD4+ T cell epitope-based heterologous prime-boost vaccination elicited potent both tumor-specific TH1 and CTL response, leading to the efficient tumor control. This strategy can also potentiate PD-1/PD-L1 immune checkpoint blockade (ICB) against cancer.
Keywords: CD4-Positive T-Lymphocytes; CD8-Positive T-Lymphocytes; Immunization; Immunotherapy; Vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors declare a conflict of interest. A patent associated with a CD4+ T cell epitope-based therapeutic vaccine has been filed (LY and RH).
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
