Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial
- PMID: 35580930
- PMCID: PMC9114861
- DOI: 10.1136/jitc-2021-004007
Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial
Abstract
Background: Melanoma is the most lethal form of skin cancer in humans. Conventional therapies have limited efficacy, and overall response is still unsatisfactory considering that immune checkpoint inhibitors induce lasting clinical responses only in a low percentage of patients. This has prompted us to develop a vaccination strategy employing the tumor antigen chondroitin sulfate proteoglycan (CSPG)4 as a target.
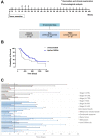
Methods: To overcome the host's unresponsiveness to the self-antigen CSPG4, we have taken advantage of the conservation of CSPG4 sequence through phylogenetic evolution, so we have used a vaccine, based on a chimeric DNA molecule encompassing both human (Hu) and dog (Do) portions of CSPG4 (HuDo-CSPG4). We have tested its safety and immunogenicity (primary objectives), along with its therapeutic efficacy (secondary outcome), in a prospective, non-randomized, veterinary clinical trial enrolling 80 client-owned dogs with surgically resected, CSPG4-positive, stage II-IV oral melanoma.
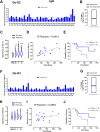
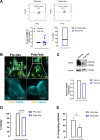
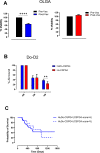
Results: Vaccinated dogs developed anti-Do-CSPG4 and Hu-CSPG4 immune response. Interestingly, the antibody titer in vaccinated dogs was significantly associated with the overall survival. Our data suggest that there may be a contribution of the HuDo-CSPG4 vaccination to the improvement of survival of vaccinated dogs as compared with controls treated with conventional therapies alone.
Conclusions: HuDo-CSPG4 adjuvant vaccination was safe and immunogenic in dogs with oral melanoma, with potential beneficial effects on the course of the disease. Thanks to the power of naturally occurring canine tumors as predictive models for cancer immunotherapy response, these data may represent a basis for the translation of this approach to the treatment of human patients with CSPG4-positive melanoma subtypes.
Keywords: Immunogenicity, Vaccine; Immunotherapy, Active; Melanoma; Translational Medical Research; Vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

Similar articles
-
Clinical evaluation of HuDo-CSPG4 DNA electroporation as adjuvant treatment for canine oral malignant melanoma: comparison of two vaccination protocols.Vet Q. 2025 Dec;45(1):1-16. doi: 10.1080/01652176.2025.2473717. Epub 2025 Mar 10. Vet Q. 2025. PMID: 40059815 Free PMC article.
-
CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA.Clin Cancer Res. 2014 Jul 15;20(14):3753-62. doi: 10.1158/1078-0432.CCR-13-3042. Epub 2014 May 29. Clin Cancer Res. 2014. PMID: 24874834 Free PMC article.
-
Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination.Vet Comp Oncol. 2017 Sep;15(3):996-1013. doi: 10.1111/vco.12239. Epub 2016 May 4. Vet Comp Oncol. 2017. PMID: 27146852 Free PMC article.
-
CSPG4: a prototype oncoantigen for translational immunotherapy studies.J Transl Med. 2017 Jul 1;15(1):151. doi: 10.1186/s12967-017-1250-4. J Transl Med. 2017. PMID: 28668095 Free PMC article. Review.
-
Cancer immunology and canine malignant melanoma: A comparative review.Vet Immunol Immunopathol. 2016 Jan;169:15-26. doi: 10.1016/j.vetimm.2015.11.003. Epub 2015 Dec 1. Vet Immunol Immunopathol. 2016. PMID: 26827834 Review.
Cited by
-
Improving Osteosarcoma Treatment: Comparative Oncology in Action.Life (Basel). 2022 Dec 14;12(12):2099. doi: 10.3390/life12122099. Life (Basel). 2022. PMID: 36556464 Free PMC article.
-
Electroporation in Translational Medicine: From Veterinary Experience to Human Oncology.Cancers (Basel). 2024 Mar 6;16(5):1067. doi: 10.3390/cancers16051067. Cancers (Basel). 2024. PMID: 38473422 Free PMC article. Review.
-
Highlighting recent achievements to advance more effective cancer immunotherapy.J Exp Clin Cancer Res. 2025 Feb 18;44(1):57. doi: 10.1186/s13046-025-03316-8. J Exp Clin Cancer Res. 2025. PMID: 39966867 Free PMC article.
-
Influence of the extent of cervical lymph node dissection and lymph nodes metastases on prognosis in a cohort of dogs with oral malignant melanoma treated by surgical resection and adjuvant anti-CSPG4 electrovaccination: a retrospective study on 77 cases.Front Vet Sci. 2025 Jul 25;12:1616419. doi: 10.3389/fvets.2025.1616419. eCollection 2025. Front Vet Sci. 2025. PMID: 40786979 Free PMC article.
-
Development of Monoclonal Antibodies Targeting Canine PD-L1 and PD-1 and Their Clinical Relevance in Canine Apocrine Gland Anal Sac Adenocarcinoma.Cancers (Basel). 2022 Dec 14;14(24):6188. doi: 10.3390/cancers14246188. Cancers (Basel). 2022. PMID: 36551672 Free PMC article.
References
-
- Shaughnessy M, Klebanov N, Tsao H. Clinical and therapeutic implications of melanoma genomics. JTGG 2018;2. 10.20517/jtgg.2018.25 - DOI
