Measuring Basic Reproduction Number to Assess Effects of Nonpharmaceutical Interventions on Nosocomial SARS-CoV-2 Transmission
- PMID: 35580960
- PMCID: PMC9239897
- DOI: 10.3201/eid2807.212339
Measuring Basic Reproduction Number to Assess Effects of Nonpharmaceutical Interventions on Nosocomial SARS-CoV-2 Transmission
Abstract
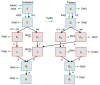
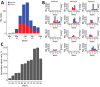
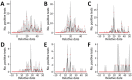
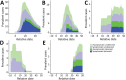
Outbreaks of SARS-CoV-2 infection frequently occur in hospitals. Preventing nosocomial infection requires insight into hospital transmission. However, estimates of the basic reproduction number (R0) in care facilities are lacking. Analyzing a closely monitored SARS-CoV-2 outbreak in a hospital in early 2020, we estimated the patient-to-patient transmission rate and R0. We developed a model for SARS-CoV-2 nosocomial transmission that accounts for stochastic effects and undetected infections and fit it to patient test results. The model formalizes changes in testing capacity over time, and accounts for evolving PCR sensitivity at different stages of infection. R0 estimates varied considerably across wards, ranging from 3 to 15 in different wards. During the outbreak, the hospital introduced a contact precautions policy. Our results strongly support a reduction in the hospital-level R0 after this policy was implemented, from 8.7 to 1.3, corresponding to a policy efficacy of 85% and demonstrating the effectiveness of nonpharmaceutical interventions.
Keywords: COVID-19; R0; SARS; SARS-CoV-2; basic reproduction number; contact precautions; coronavirus; coronavirus disease; iterative filtering; long-term care facility; nosocomial infection; patient-to-patient transmission; respiratory infections; severe acute respiratory syndrome coronavirus 2; statistical inference; stochastic modelling; transmission rate; viruses; zoonoses.
Figures
References
-
- Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. ; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–35. 10.1016/S0140-6736(21)00790-X - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

