Study protocol for a single-centre randomised controlled trial to investigate the effect of lung recruitment in paediatric patients after cardiac surgery
- PMID: 35580972
- PMCID: PMC9115041
- DOI: 10.1136/bmjopen-2022-063278
Study protocol for a single-centre randomised controlled trial to investigate the effect of lung recruitment in paediatric patients after cardiac surgery
Abstract
Introduction: A number of published studies have revealed that lung recruitment can improve oxygenation, shorten the duration of mechanical ventilation (MV) and decrease mortality in adults with acute hypoxaemic respiratory failure, especially patients with acute respiratory distress syndrome. However, few articles have assessed lung recruitment in paediatric patients, especially after cardiac surgery. This clinical trial aimed to determine whether lung recruitment can reduce the duration of MV in paediatric patients with hypoxaemic respiratory failure after cardiac surgery.
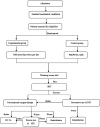
Method and analysis: In this trial, we will randomly assign 234 paediatric patients (aged 28 days to 14 years) within 72 hours after cardiac surgery with an arterial oxygen tension (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2) of <300 to either a lung recruitment group or a conventional group. The primary endpoint will be the duration of MV. The secondary endpoints will be ventilator-free days, PaO2/FiO2, respiratory system compliance, duration of non-invasive ventilation, reintubation rate, length of intensive care unit stay, length of hospital stay, occurrence of serious adverse events (barotrauma, persistent hypotension and arrhythmia), postoperative pulmonary complications.
Ethics and dissemination: The ethics committee of West China Hospital of Sichuan University granted ethics approval for this study (20 August 2019). The results will be published in peer-reviewed journals and presented at conferences.
Trial registration number: ChiCTR1900025990.
Keywords: Cardiac surgery; Paediatric cardiac surgery; Paediatric intensive & critical care; Protocols & guidelines.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
