Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival
- PMID: 35580994
- PMCID: PMC9427709
- DOI: 10.1111/nyas.14798
Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival
Abstract
Epithelial cells are the first line of mucosal defense. In the intestine, a single layer of epithelial cells must establish a selectively permeable barrier that supports nutrient absorption and waste secretion while preventing the leakage of potentially harmful luminal materials. Key to this is the tight junction, which seals the paracellular space and prevents unrestricted leakage. The tight junction is a protein complex established by interactions between members of the claudin, zonula occludens, and tight junction-associated MARVEL protein (TAMP) families. Claudins form the characteristic tight junction strands seen by freeze-fracture microscopy and create paracellular channels, but the functions of ZO-1 and occludin, founding members of the zonula occludens and TAMP families, respectively, are less well defined. Recent studies have revealed that these proteins have essential noncanonical (nonbarrier) functions that allow them to regulate epithelial apoptosis and proliferation, facilitate viral entry, and organize specialized epithelial structures. Surprisingly, neither is required for intestinal barrier function or overall health in the absence of exogenous stressors. Here, we provide a brief overview of ZO-1 and occludin canonical (barrier-related) functions, and a more detailed examination of their noncanonical functions.
Keywords: actin; barrier; claudin; intestine; permeability.
© 2022 New York Academy of Sciences.
Conflict of interest statement
Competing interests
J.R.T. is a founder and shareholder of Thelium Therapeutics and has served is a consultant for Entrinsic, Immunic, and Kallyope.
Figures
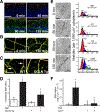

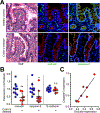
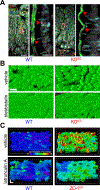


References
-
- Van Itallie CM & Anderson JM 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol 68: 403–429. - PubMed
-
- Turner JR 2009. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 9: 799–809. - PubMed
-
- Marchiando AM, Graham WV & Turner JR 2010. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 5: 119–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

