Cell Death in Development
- PMID: 35581003
- PMCID: PMC9121905
- DOI: 10.1101/cshperspect.a041095
Cell Death in Development
Figures

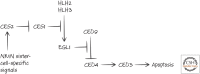
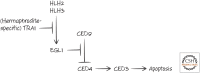
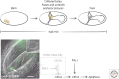
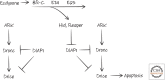


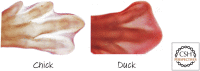

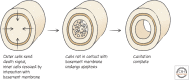
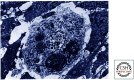


References
FIGURE CREDITS
-
- Kerr JFR, Harmon B, Searle J. 1974. An electron-microscope study of cell deletion in the anuran tadpole tail during spontaneous metamorphosis with special reference to apoptosis of striated muscle fibres. J Cell Sci 14: 571–585. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
