Analysis of rhodopsin G protein-coupled receptor orthologs reveals semiochemical peptides for parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) interplay
- PMID: 35581232
- PMCID: PMC9114394
- DOI: 10.1038/s41598-022-11996-x
Analysis of rhodopsin G protein-coupled receptor orthologs reveals semiochemical peptides for parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) interplay
Abstract
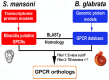
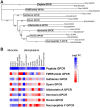
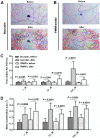
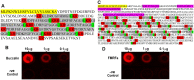
Schistosomiasis is a medically significant disease caused by helminth parasites of the genus Schistosoma. The schistosome life cycle requires chemically mediated interactions with an intermediate (aquatic snail) and definitive (human) host. Blocking parasite development within the snail stage requires improved understanding of the interactions between the snail host and the Schistosoma water-borne free-living form (miracidium). Innovations in snail genomics and aquatic chemical communication provide an ideal opportunity to explore snail-parasite coevolution at the molecular level. Rhodopsin G protein-coupled receptors (GPCRs) are of particular interest in studying how trematode parasites navigate towards their snail hosts. The potential role of GPCRs in parasites makes them candidate targets for new antihelminthics that disrupt the intermediate host life-cycle stages, thus preventing subsequent human infections. A genomic-bioinformatic approach was used to identify GPCR orthologs between the snail Biomphalaria glabrata and miracidia of its obligate parasite Schistosoma mansoni. We show that 8 S. mansoni rhodopsin GPCRs expressed within the miracidial stage share overall amino acid similarity with 8 different B. glabrata rhodopsin GPCRs, particularly within transmembrane domains, suggesting conserved structural features. These GPCRs include an orphan peptide receptor as well as several with strong sequence homologies with rhabdomeric opsin receptors, a serotonin receptor, a sulfakinin (SK) receptor, an allatostatin-A (buccalin) receptor and an FMRFamide receptor. Buccalin and FMRFa peptides were identified in water conditioned by B. glabrata, and we show synthetic buccalin and FMRFa can stimulate significant rates of change of direction and turn-back responses in S. mansoni miracidia. Ortholog GPCRs were identified in S. mansoni miracidia and B. glabrata. These GPCRs may detect similar ligands, including snail-derived odorants that could facilitate miracidial host finding. These results lay the foundation for future research elucidating the mechanisms by which GPCRs mediate host finding which can lead to the potential development of novel anti-schistosome interventions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2013;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Ittiprasert, W., Myers, J., Odoemelam, E. C., Raghavan, N., Lewis, F., Bridger, J. M., & Knight, M. Advances in the Genomics and Proteomics of the Freshwater Intermediate Snail Host of Schistosoma mansoni, Biomphalaria glabrata. In: Biomphalaria Snails and Larval Trematodes. edn.: Springer; 2011: 191–213.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

