Periodontal tissue regeneration by transplantation of autologous adipose tissue-derived multi-lineage progenitor cells
- PMID: 35581234
- PMCID: PMC9114023
- DOI: 10.1038/s41598-022-11986-z
Periodontal tissue regeneration by transplantation of autologous adipose tissue-derived multi-lineage progenitor cells
Abstract
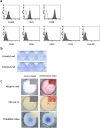
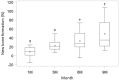
Periodontitis is a chronic inflammatory disease that destroys tooth-supporting periodontal tissue. Current periodontal regenerative therapies have unsatisfactory efficacy; therefore, periodontal tissue engineering might be established by developing new cell-based therapies. In this study, we evaluated the safety and efficacy of adipose tissue-derived multi-lineage progenitor cells (ADMPC) autologous transplantation for periodontal tissue regeneration in humans. We conducted an open-label, single-arm exploratory phase I clinical study in which 12 periodontitis patients were transplanted with autologous ADMPCs isolated from subcutaneous adipose tissue. Each patient underwent flap surgery during which autologous ADMPCs were transplanted into the bone defect with a fibrin carrier material. Up to 36 weeks after transplantation, we performed a variety of clinical examinations including periodontal tissue inspection and standardized dental radiographic analysis. A 36-week follow-up demonstrated no severe transplantation-related adverse events in any cases. ADMPC transplantation reduced the probing pocket depth, improved the clinical attachment level, and induced neogenesis of alveolar bone. Therapeutic efficiency was observed in 2- or 3-walled vertical bone defects as well as more severe periodontal bone defects. These results suggest that autologous ADMPC transplantation might be an applicable therapy for severe periodontitis by inducing periodontal regeneration.
© 2022. The Author(s).
Conflict of interest statement
H.O. is a director and A.M. is a Scientific Advisor of Adipo Medical Technology Inc. The other authors indicated no potential conflicts of interest.
Figures



Similar articles
-
Periodontal Tissue Regeneration by Transplantation of Autologous Adipose Tissue-Derived Multi-Lineage Progenitor Cells With Carbonate Apatite.Cell Transplant. 2023 Jan-Dec;32:9636897231198296. doi: 10.1177/09636897231198296. Cell Transplant. 2023. PMID: 37710973 Free PMC article.
-
Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in vivo.Sci Rep. 2019 Jan 29;9(1):921. doi: 10.1038/s41598-018-37528-0. Sci Rep. 2019. PMID: 30696909 Free PMC article.
-
A novel marginal periosteal pedicle graft as an autogenous guided tissue membrane for the treatment of intrabony periodontal defects.J Int Acad Periodontol. 2008 Oct;10(4):106-17. J Int Acad Periodontol. 2008. PMID: 19055224 Clinical Trial.
-
Periodontal regeneration - intrabony defects: a consensus report from the AAP Regeneration Workshop.J Periodontol. 2015 Feb;86(2 Suppl):S105-7. doi: 10.1902/jop.2015.140378. Epub 2014 Oct 15. J Periodontol. 2015. PMID: 25315019
-
Recent advances in horizontal alveolar bone regeneration.Biomed Mater. 2023 Jul 24;18(5). doi: 10.1088/1748-605X/acd672. Biomed Mater. 2023. PMID: 37196651 Review.
Cited by
-
Application of mesenchymal stem/stromal cells in periodontal regeneration: Opportunities and challenges.Jpn Dent Sci Rev. 2024 Dec;60:95-108. doi: 10.1016/j.jdsr.2024.01.001. Epub 2024 Jan 25. Jpn Dent Sci Rev. 2024. PMID: 38314143 Free PMC article. Review.
-
Categories, applications, and potential of stem cells in bone regeneration: an overview.Front Med (Lausanne). 2025 Aug 20;12:1606100. doi: 10.3389/fmed.2025.1606100. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40909447 Free PMC article. Review.
-
From mitochondria to immune networks: new mesenchymal stem cell strategies to treat periodontitis.Stem Cell Res Ther. 2025 Aug 29;16(1):470. doi: 10.1186/s13287-025-04619-5. Stem Cell Res Ther. 2025. PMID: 40877977 Free PMC article. Review.
-
Periodontal Tissue Regeneration by Transplantation of Autologous Adipose Tissue-Derived Multi-Lineage Progenitor Cells With Carbonate Apatite.Cell Transplant. 2023 Jan-Dec;32:9636897231198296. doi: 10.1177/09636897231198296. Cell Transplant. 2023. PMID: 37710973 Free PMC article.
-
CH02 peptide-stimulated periodontal ligament cells enhance periodontal defect repair in rats.BMC Oral Health. 2025 Jul 3;25(1):1078. doi: 10.1186/s12903-025-06393-5. BMC Oral Health. 2025. PMID: 40611174 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

