Unveiling host-guest-solvent interactions in solution by identifying highly unstable host-guest configurations in thermal non-equilibrium gas phase
- PMID: 35581255
- PMCID: PMC9114120
- DOI: 10.1038/s41598-022-12226-0
Unveiling host-guest-solvent interactions in solution by identifying highly unstable host-guest configurations in thermal non-equilibrium gas phase
Abstract
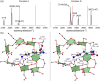
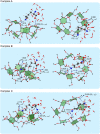
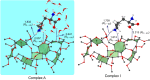
We propose a novel scheme of examining the host-guest-solvent interactions in solution from their gas phase structures. By adopting the permethylated β-cyclodextrin (perm β-CD)-protonated L-Lysine non-covalent complex as a prototypical system, we present the infrared multiple photon dissociation (IRMPD) spectrum of the gas phase complex produced by electrospray ionization technique. In order to elucidate the structure of perm β-CD)/LysH+ complex in the gas phase, we carry out quantum chemical calculations to assign the two strong peaks at 3,340 and 3,560 cm-1 in the IRMPD spectrum, finding that the carboxyl forms loose hydrogen bonding with the perm β-CD, whereas the ammonium group of L-Lysine is away from the perm β-CD unit. By simulating the structures of perm β-CD/H+/L-Lysine complex in solution using the supramolecule/continuum model, we find that the extremely unstable gas phase structure corresponds to the most stable conformer in solution.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown 8/Protonated Serine Noncovalent Complexes in the Gas Phase vs in Solution: Quantum Chemical Analysis.ACS Omega. 2024 May 23;9(22):23793-23801. doi: 10.1021/acsomega.4c01782. eCollection 2024 Jun 4. ACS Omega. 2024. PMID: 38854571 Free PMC article.
-
Delineating Host-Guest-Solvent Interactions in Solution from Gas-Phase Host-Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase.Molecules. 2025 Apr 11;30(8):1723. doi: 10.3390/molecules30081723. Molecules. 2025. PMID: 40333634 Free PMC article.
-
Infrared multiple photon dissociation spectroscopy and density functional theory (DFT) studies of protonated permethylated β-cyclodextrin-water non-covalent complexes.Phys Chem Chem Phys. 2014 May 14;16(18):8376-83. doi: 10.1039/c3cp54841d. Phys Chem Chem Phys. 2014. PMID: 24658048
-
Chiral differentiation of d- and l-isoleucine using permethylated β-cyclodextrin: infrared multiple photon dissociation spectroscopy, ion-mobility mass spectrometry, and DFT calculations.Phys Chem Chem Phys. 2018 Dec 12;20(48):30428-30436. doi: 10.1039/c8cp05617j. Phys Chem Chem Phys. 2018. PMID: 30499999
-
Noncovalent Complexes of Cyclodextrin with Small Organic Molecules: Applications and Insights into Host-Guest Interactions in the Gas Phase and Condensed Phase.Molecules. 2020 Sep 4;25(18):4048. doi: 10.3390/molecules25184048. Molecules. 2020. PMID: 32899713 Free PMC article. Review.
Cited by
-
Structural Modification and Biological Activity of Polysaccharides.Molecules. 2023 Jul 14;28(14):5416. doi: 10.3390/molecules28145416. Molecules. 2023. PMID: 37513287 Free PMC article. Review.
-
The Effect of Alkali Iodide Salts in the Inclusion Process of Phenolphthalein in β-Cyclodextrin: A Spectroscopic and Theoretical Study.Molecules. 2023 Jan 23;28(3):1147. doi: 10.3390/molecules28031147. Molecules. 2023. PMID: 36770813 Free PMC article.
-
Mass Spectrometry, Ion Mobility Separation and Molecular Modelling: A Powerful Combination for the Structural Characterisation of Substituted Cyclodextrins Mixtures.Int J Mol Sci. 2022 Nov 1;23(21):13352. doi: 10.3390/ijms232113352. Int J Mol Sci. 2022. PMID: 36362136 Free PMC article.
-
Thermodynamic Reversal and Structural Correlation of 24-Crown-8/Protonated Tryptophan and 24-Crown 8/Protonated Serine Noncovalent Complexes in the Gas Phase vs in Solution: Quantum Chemical Analysis.ACS Omega. 2024 May 23;9(22):23793-23801. doi: 10.1021/acsomega.4c01782. eCollection 2024 Jun 4. ACS Omega. 2024. PMID: 38854571 Free PMC article.
-
Delineating Host-Guest-Solvent Interactions in Solution from Gas-Phase Host-Guest Configurations: Thermodynamic Reversal and Structural Correlation of 24-Crown-8/H+/Diaminopropanol Non-Covalent Complexes in Aqueous Solution vs. in the Gas Phase.Molecules. 2025 Apr 11;30(8):1723. doi: 10.3390/molecules30081723. Molecules. 2025. PMID: 40333634 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

