Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis
- PMID: 35581292
- PMCID: PMC9622891
- DOI: 10.1038/s41401-022-00917-3
Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis
Erratum in
-
Author Correction: Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis.Acta Pharmacol Sin. 2022 Nov;43(11):3011-3012. doi: 10.1038/s41401-022-00969-5. Acta Pharmacol Sin. 2022. PMID: 35974170 Free PMC article. No abstract available.
-
Author Correction: Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing KEAP1/NRF2 and CBS-regulated ferroptosis.Acta Pharmacol Sin. 2024 Oct;45(10):2226-2227. doi: 10.1038/s41401-024-01271-2. Acta Pharmacol Sin. 2024. PMID: 38740905 Free PMC article. No abstract available.
Abstract
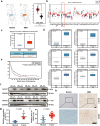
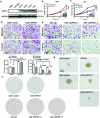
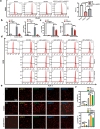
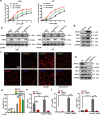
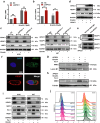
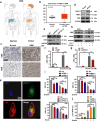
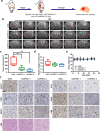
Glioblastoma (GBM), a malignant brain tumor, is a world-wide health problem because of its poor prognosis and high rates of recurrence and mortality. Apolipoprotein C1 (APOC1) is the smallest of apolipoproteins, implicated in many diseases. Recent studies have shown that APOC1 promotes tumorigenesis and development of several types of cancer. In this study we investigated the role of APOC1 in GBM tumorigenesis. Using in silico assays we showed that APOC1 was highly expressed in GBM tissues and its expression was closely related to GBM progression. We showed that APOC1 protein expression was markedly increased in four GBM cell lines (U251, U138, A172 and U87) compared to the normal brain glia cell lines (HEB, HA1800). In U251 cells, overexpression of APOC1 promoted cell proliferation, migration, invasion and colony information, which was reversed by APOC1 knockdown. APOC1 knockdown also markedly inhibited the growth of GBM xenografts in the ventricle of nude mice. We further demonstrated that APOC1 reduced ferroptosis by inhibiting KEAP1, promoting nuclear translocation of NRF2 and increasing expression of HO-1 and NQO1 in GBM cells. APOC1 also induced ferroptosis resistance by increasing cystathionine beta-synthase (CBS) expression, which promoted trans-sulfuration and increased GSH synthesis, ultimately leading to an increase in glutathione peroxidase-4 (GPX4). Thus, APOC1 plays a key role in GBM tumorigenesis, conferring resistance to ferroptosis, and may be a promising therapeutic target for GBM.
Keywords: APOC1; CBS; NRF2; ferroptosis; glioblastoma; lipid ROS; tumorigenesis.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

