Blue-light fundus autofluorescence imaging of pigment epithelial detachments
- PMID: 35581370
- PMCID: PMC10102186
- DOI: 10.1038/s41433-022-02076-5
Blue-light fundus autofluorescence imaging of pigment epithelial detachments
Abstract
Background: Pigment epithelial detachments (PEDs) occur in association with various chorioretinal diseases. With respect to the broad clinical spectrum of PEDs we describe fundus autofluorescence (FAF) characteristics of PEDs.
Methods: Ninety-three eyes of 66 patients (mean age 71.9 ± 11.1) with uni- or bilateral PED ( ≥ 350 µm) were included in a retrospective cross-sectional study. PEDs were secondary to age-related macular degeneration (n = 79), central serous chorioretinopathy (n = 7), polypoidal choroidal vasculopathy (n = 2), pattern dystrophy (n = 3) or idiopathic PED (n = 2). FAF images were recorded using confocal scanning laser ophthalmoscopy (488 nm excitation wavelength, detection of emission >500 nm). Diagnosis of PED was confirmed using spectral-domain optical coherence tomography. A qualitative FAF grading system was established, and grading was performed by two independent readers.
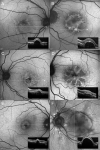
Results: PEDs showed highly variable characteristics on FAF imaging. FAF within the area of PED was found to be irregular/granular (n = 59, 63.4%), increased (n = 28, 30.1%), decreased (n = 3, 3.2 %), or normal (n = 3, 3.2%). Accompanying FAF changes included condensation of macular pigment (n = 67, 72.0%), focally increased FAF at the PED apex (n = 14, 15.1%) or elsewhere (n = 52, 55.9%), focally decreased FAF (n = 23, 24.7%), a cartwheel-like pattern (n = 10, 10.8%), a doughnut sign (n = 6, 6.5%), and a halo of decreased FAF encircling the PED (completely n = 20, 21.5% or incompletely n = 20, 21.5%).
Conclusions: PEDs show a variety of abnormal patterns on FAF imaging. These changes in FAF signals may be secondary to morphological and metabolic alterations within corresponding retinal layers and do not necessarily correspond with the underlying PED subtype or a specific pathology.
© 2022. The Author(s).
Conflict of interest statement
Almut Bindewald-Wittich: reports non-financial support from Heidelberg Engineering outside the submitted work. Joanna Dolar-Szczasny: No conflict of interest to declare. Sandrine H. Kuenzel: No conflict of interest to declare. Leon von der Emde: No conflict of interest to declare. Maximilian Pfau: reports non-financial support from Carl Zeiss Meditec, Centervue; Heidelberg Engineering and Optos, outside the submitted work. Robert Rejdak: No conflict of interest to declare. Steffen Schmitz-Valckenberg: reports grants from Kubota Vision, grants and personal fees from Apellis, grants and personal fees from Novartis, grants and personal fees from Bioeq/Formycon, non-financial support from Carl Zeiss MediTec AG, personal fees and non-financial support from Heidelberg Engineering, grants from Katairo, non-financial support from Optos, personal fees from Oxurion, grants from Pixium, grants and personal fees from Roche, grants from SparingVision, outside the submitted work, Founder STZ GRADE Reading Center outside the submitted work. Thomas Ach: reports personal fees from Heidelberg Engineering, grants and personal fees from Novartis, personal fees from Roche, outside the submitted work. Jens Dreyhaupt: No conflicts of interest to declare. Frank G. Holz: reports grants and personal fees from Heidelberg Engineering, grants and personal fees from Optos, grants from Zeiss, during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from Bayer Healthcare, grants and personal fees from Genentech, grants and personal fees from Acucela, Kanghong, personal fees from Boehringer Ingelheim, grants and personal fees from Alcon, grants and personal fees from Allergan, outside the submitted work.
Figures

Similar articles
-
Central serous chorioretinopathy fundus autofluorescence comparison with two different confocal scanning laser ophthalmoscopes.Graefes Arch Clin Exp Ophthalmol. 2015 Dec;253(12):2121-7. doi: 10.1007/s00417-015-2958-6. Epub 2015 Feb 18. Graefes Arch Clin Exp Ophthalmol. 2015. PMID: 25690981
-
Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) in Eyes With Pigment Epithelial Detachments Due to Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2019 Jul 1;60(8):3054-3063. doi: 10.1167/iovs.19-26835. Invest Ophthalmol Vis Sci. 2019. PMID: 31348823 Free PMC article.
-
Retinal pigment epithelium apertures as a late complication of longstanding serous pigment epithelium detachments in chronic central serous chorioretinopathy.Eye (Lond). 2019 Dec;33(12):1871-1876. doi: 10.1038/s41433-019-0505-0. Epub 2019 Jul 2. Eye (Lond). 2019. PMID: 31267093 Free PMC article.
-
Multimodal imaging of pigment epithelial detachment: a guide to evaluation.Retina. 2013 Oct;33(9):1735-62. doi: 10.1097/IAE.0b013e3182993f66. Retina. 2013. PMID: 23873168 Review.
-
[Pathophysiology of macular diseases--morphology and function].Nippon Ganka Gakkai Zasshi. 2011 Mar;115(3):238-74; discussion 275. Nippon Ganka Gakkai Zasshi. 2011. PMID: 21476310 Review. Japanese.
Cited by
-
Impact of lens autofluorescence and opacification on retinal imaging.BMJ Open Ophthalmol. 2024 Apr 29;9(1):e001628. doi: 10.1136/bmjophth-2023-001628. BMJ Open Ophthalmol. 2024. PMID: 38684375 Free PMC article.
-
Integrating Machine Learning and Traditional Survival Analysis to Identify Key Predictors of Foveal Involvement in Geographic Atrophy.Invest Ophthalmol Vis Sci. 2024 May 1;65(5):10. doi: 10.1167/iovs.65.5.10. Invest Ophthalmol Vis Sci. 2024. PMID: 38709525 Free PMC article.
-
Unified modeling of photothermal and photochemical damage.Front Ophthalmol (Lausanne). 2024 Aug 19;4:1408869. doi: 10.3389/fopht.2024.1408869. eCollection 2024. Front Ophthalmol (Lausanne). 2024. PMID: 39224466 Free PMC article.
-
Blue Light and Green Light Fundus Autofluorescence, Complementary to Optical Coherence Tomography, in Age-Related Macular Degeneration Evaluation.Diagnostics (Basel). 2025 Jul 2;15(13):1688. doi: 10.3390/diagnostics15131688. Diagnostics (Basel). 2025. PMID: 40647687 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

