Pulsed-Focused Ultrasound Provides Long-Term Suppression of Epileptiform Bursts in the Kainic Acid-Induced Epilepsy Rat Model
- PMID: 35581489
- PMCID: PMC9587190
- DOI: 10.1007/s13311-022-01250-7
Pulsed-Focused Ultrasound Provides Long-Term Suppression of Epileptiform Bursts in the Kainic Acid-Induced Epilepsy Rat Model
Abstract
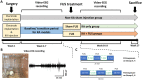
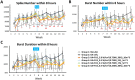
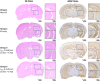
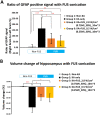
Focused ultrasound (FUS) has potential utility for modulating regional brain excitability and possibly aiding seizure control; however, the duration of any beneficial effect is unknown. This study explores the efficacy and time course of a short series of pulsed FUS in suppressing EEG epileptiform spikes/bursts in a kainic acid (KA) animal model of temporal lobe epilepsy. Forty-four male Sprague-Dawley rats were recorded for 14 weeks with EEG while software calculated EEG numbers of epileptiform spikes and bursts (≥ 3 spikes/s). Four regimens of FUS given in a single session at week 7 were evaluated, with mechanical index (MI) ranging from 0.25 to 0.75, intensity spatial peak temporal average (ISPTA) from 0.1 to 2.8 W per cm2, duty cycle from 1 to 30%, and three consecutive pulse trains for 5 or 10 min each. Controls included sham injections in four and KA without FUS in eleven animals. Histological analysis investigated tissue effects. All animals receiving KA evidenced EEG spikes, averaging 10,378 ± 1651 spikes per 8 h and 1255 ± 199 bursts per 8 h by weeks 6-7. The KA-only group showed a 30% of increase in spikes and bursts by week 14. Compared to the KA-only group, spike counts were reduced by about 25%, burst counts by about 33%, and burst durations by about 50% with FUS. Behavioral seizures were not analyzed, but electrographic seizures longer than 10 s declined up to 70% after some FUS regimens. Repeated-measure ANOVA showed a significant effect of higher intensity and longer sonication duration FUS treatment using 0.75-MI, ISPTA 2.8 W/cm2, 30% duty cycle for 10-min sonications (group effect, F (4, 15) = 6.321, p < 0.01; interaction effect, F (44, 165) = 1.726, p < 0.01), with the hippocampal protective effect lasting to week 14, accompanied by decreased inflammation and gliosis effect. In contrast, spike and burst suppression were achieved using an FUS regimen with 0.25-MI ISPTA 0.5 W/cm2, 30% duty cycle for 10-min sonications. This regimen reduced inflammation and gliosis at weeks 8-14 and protected hippocampal tissue. This study demonstrates that low-intensity pulsed ultrasound can modulate epileptiform activity for up to 7 weeks and, if replicated in the clinical setting, might be a practical treatment for epilepsy.
Keywords: Focused ultrasound; Kainic acid model; Neuromodulation; Temporal lobe epilepsy.
© 2022. The Author(s).
Figures




Similar articles
-
Pulsed Focused Ultrasound Reduces Hippocampal Volume Loss and Improves Behavioral Performance in the Kainic Acid Rat Model of Epilepsy.Neurotherapeutics. 2023 Mar;20(2):502-517. doi: 10.1007/s13311-023-01363-7. Epub 2023 Mar 14. Neurotherapeutics. 2023. PMID: 36917440 Free PMC article.
-
Focused ultrasound suppresses pentylenetetrazol-induced epileptiform activity in rats and alters connectivity measured by functional MRI.Sci Rep. 2025 Aug 12;15(1):29551. doi: 10.1038/s41598-025-15305-0. Sci Rep. 2025. PMID: 40797064 Free PMC article.
-
Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo.Brain Stimul. 2020 Jan-Feb;13(1):35-46. doi: 10.1016/j.brs.2019.09.011. Epub 2019 Sep 24. Brain Stimul. 2020. PMID: 31575487
-
Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity.BMC Neurosci. 2011 Mar 6;12:23. doi: 10.1186/1471-2202-12-23. BMC Neurosci. 2011. PMID: 21375781 Free PMC article.
-
Focused ultrasound for treatment of epilepsy: a systematic review and meta-analysis of preclinical and clinical studies.Neurosurg Rev. 2024 Nov 10;47(1):839. doi: 10.1007/s10143-024-03078-5. Neurosurg Rev. 2024. PMID: 39521750
Cited by
-
Pulsed Focused Ultrasound Reduces Hippocampal Volume Loss and Improves Behavioral Performance in the Kainic Acid Rat Model of Epilepsy.Neurotherapeutics. 2023 Mar;20(2):502-517. doi: 10.1007/s13311-023-01363-7. Epub 2023 Mar 14. Neurotherapeutics. 2023. PMID: 36917440 Free PMC article.
-
Transcranial focused ultrasound-mediated unbinding of phenytoin from plasma proteins for suppression of chronic temporal lobe epilepsy in a rodent model.Sci Rep. 2023 Mar 13;13(1):4128. doi: 10.1038/s41598-023-31383-4. Sci Rep. 2023. PMID: 36914775 Free PMC article.
-
Ultrasonic therapies for seizures and drug-resistant epilepsy.Front Neurol. 2023 Dec 12;14:1301956. doi: 10.3389/fneur.2023.1301956. eCollection 2023. Front Neurol. 2023. PMID: 38162441 Free PMC article. Review.
-
Transcranial low-intensity ultrasound stimulation for treating central nervous system disorders: A promising therapeutic application.Front Neurol. 2023 Mar 8;14:1117188. doi: 10.3389/fneur.2023.1117188. eCollection 2023. Front Neurol. 2023. PMID: 36970512 Free PMC article. Review.
-
Advances in using ultrasound to regulate the nervous system.Neurol Sci. 2024 Jul;45(7):2997-3006. doi: 10.1007/s10072-024-07426-7. Epub 2024 Mar 4. Neurol Sci. 2024. PMID: 38436788 Review.
References
-
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. - PubMed
-
- Falconer MA, Serafetinides EA, Corsellis JA. Etiology and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233–248. - PubMed
-
- Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26(1):141–150. - PubMed

