HIV-1 drug resistance genotyping success rates and correlates of Dried-blood spots and plasma specimen genotyping failure in a resource-limited setting
- PMID: 35581555
- PMCID: PMC9112432
- DOI: 10.1186/s12879-022-07453-9
HIV-1 drug resistance genotyping success rates and correlates of Dried-blood spots and plasma specimen genotyping failure in a resource-limited setting
Abstract
Background: HIV-1 drug resistance genotyping is critical to the monitoring of antiretroviral treatment. Data on HIV-1 genotyping success rates of different laboratory specimen types from multiple sources is still scarce.
Methods: In this cross-sectional study, we determined the laboratory genotyping success rates (GSR) and assessed the correlates of genotyping failure of 6837 unpaired dried blood spot (DBS) and plasma specimens. Specimens from multiple studies in a resource-constrained setting were analysed in our laboratory between 2016 and 2019.
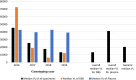
Results: We noted an overall GSR of 65.7% and specific overall GSR for DBS and plasma of 49.8% and 85.9% respectively. The correlates of genotyping failure were viral load (VL) < 10,000 copies/mL (aOR 0.3 95% CI: 0.24-0.38; p < 0.0001), lack of viral load testing prior to genotyping (OR 0.85 95% CI: 0.77-0.94; p = 0.002), use of DBS specimens (aOR 0.10 95% CI: 0.08-0.14; p < 0.0001) and specimens from routine clinical diagnosis (aOR 1.4 95% CI: 1.10-1.75; p = 0.005).
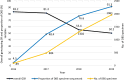
Conclusions: We report rapidly decreasing HIV-1 genotyping success rates between 2016 and 2019 with increased use of DBS specimens for genotyping and note decreasing median viral loads over the years. We recommend improvement in DBS handling, pre-genotyping viral load testing to screen samples to enhance genotyping success and the development of more sensitive assays with well-designed primers to genotype specimens with low or undetectable viral load, especially in this era where virological suppression rates are rising due to increased antiretroviral therapy roll-out.
Keywords: DBS; Genotypic resistance testing; HIV-1; Plasma; Resource-limited settings; Success rates.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- UNAIDS. Fact sheet-Global HIV Statistics [Internet]. 2020 [cited 2020 Oct 11]. Available from: http://aidsinfo.unaids.org.
-
- Ministry of Health. Consolidated Guidelines for the Prevention and Treatment of HIV and AIDS in Uganda [Internet]. MOH; 2020. Available from: https://elearning.idi.co.ug/pluginfile.php/5675/mod_page/content.
-
- Gregson J, Tang M, Ndembi N, Hamers RL, Rhee S-Y, Marconi VC, et al. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16:565–575. doi: 10.1016/S1473-3099(15)00536-8. - DOI - PMC - PubMed
-
- Ssemwanga D, Lihana RW, Ugoji C, Abimiku A, Nkengasong J, Dakum P, et al. Update on HIV-1 acquired and transmitted drug resistance in Africa. AIDS Rev. 2015;17:3–20. - PubMed
-
- Aves T, Tambe J, Siemieniuk RA, Mbuagbaw L. Antiretroviral resistance testing in HIV‐positive people. Cochrane Database Syst Rev [Internet]. 2018 [cited 2020 Oct 12];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6517236/. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

