Early short course of neuromuscular blocking agents in patients with COVID-19 ARDS: a propensity score analysis
- PMID: 35581612
- PMCID: PMC9112652
- DOI: 10.1186/s13054-022-03983-5
Early short course of neuromuscular blocking agents in patients with COVID-19 ARDS: a propensity score analysis
Abstract
Background: The role of neuromuscular blocking agents (NMBAs) in coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS) is not fully elucidated. Therefore, we aimed to investigate in COVID-19 patients with moderate-to-severe ARDS the impact of early use of NMBAs on 90-day mortality, through propensity score (PS) matching analysis.
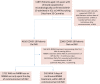
Methods: We analyzed a convenience sample of patients with COVID-19 and moderate-to-severe ARDS, admitted to 244 intensive care units within the COVID-19 Critical Care Consortium, from February 1, 2020, through October 31, 2021. Patients undergoing at least 2 days and up to 3 consecutive days of NMBAs (NMBA treatment), within 48 h from commencement of IMV were compared with subjects who did not receive NMBAs or only upon commencement of IMV (control). The primary objective in the PS-matched cohort was comparison between groups in 90-day in-hospital mortality, assessed through Cox proportional hazard modeling. Secondary objectives were comparisons in the numbers of ventilator-free days (VFD) between day 1 and day 28 and between day 1 and 90 through competing risk regression.
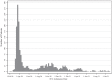
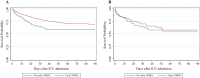
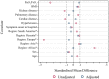
Results: Data from 1953 patients were included. After propensity score matching, 210 cases from each group were well matched. In the PS-matched cohort, mean (± SD) age was 60.3 ± 13.2 years and 296 (70.5%) were male and the most common comorbidities were hypertension (56.9%), obesity (41.1%), and diabetes (30.0%). The unadjusted hazard ratio (HR) for death at 90 days in the NMBA treatment vs control group was 1.12 (95% CI 0.79, 1.59, p = 0.534). After adjustment for smoking habit and critical therapeutic covariates, the HR was 1.07 (95% CI 0.72, 1.61, p = 0.729). At 28 days, VFD were 16 (IQR 0-25) and 25 (IQR 7-26) in the NMBA treatment and control groups, respectively (sub-hazard ratio 0.82, 95% CI 0.67, 1.00, p = 0.055). At 90 days, VFD were 77 (IQR 0-87) and 87 (IQR 0-88) (sub-hazard ratio 0.86 (95% CI 0.69, 1.07; p = 0.177).
Conclusions: In patients with COVID-19 and moderate-to-severe ARDS, short course of NMBA treatment, applied early, did not significantly improve 90-day mortality and VFD. In the absence of definitive data from clinical trials, NMBAs should be indicated cautiously in this setting.
Keywords: COVID-19; Intensive care unit; Mechanical ventilation; Neuromuscular blocking agent; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
A/Prof Li Bassi received research support from Fisher & Paykel outside the submitted work. Prof. Dalton consults with Innovative ECMO Concepts, Abiomed and Instrumentation Labs, which does not affect the current work. Prof. Brodie receives research support from ALung Technologies, and he has been on the medical advisory boards for Baxter, Abiomed, Xenios, and Hemovent. A/Prof Fan reports personal fees from ALung Technologies, Baxter, Fresenius Medical Care, Getinge, and MC3 Cardiopulmonary outside the submitted work. Prof. Laffey reports consulting fees from Baxter and Cala Medical, both outside the submitted work. Prof. Nichol is supported by a Health Research Board of Ireland Award (CTN-2014-012). Prof. Fraser receives research support from Fisher & Paykel outside the submitted work. Prof. Grasselli reports personal fees from Draeger Medical, Biotest, Getinge, Fisher & Paykel, and MSD outside the submitted work.
Figures
References
-
- COVID-19 Map-Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2020 Jun 7]. Available from: https://coronavirus.jhu.edu/map.html.
-
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:583–862. doi: 10.1016/S2213-2600(20)30316-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
- Andalusian Health Repository - access to free full text
- Archivio Istituzionale della Ricerca Unimi - Access Free Full Text
- BioMed Central
- Europe PubMed Central
- PubMed Central
- University of Turin Instituional Repository AperTO - Free full text
- eScholarship, University of California - Access Free Full Text
Miscellaneous

