Functional implications of microvascular heterogeneity for oxygen uptake and utilization
- PMID: 35581743
- PMCID: PMC9114652
- DOI: 10.14814/phy2.15303
Functional implications of microvascular heterogeneity for oxygen uptake and utilization
Abstract
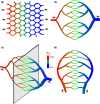
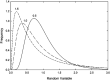
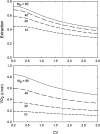
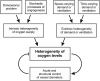
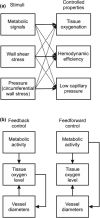
In the vascular system, an extensive network structure provides convective and diffusive transport of oxygen to tissue. In the microcirculation, parameters describing network structure, blood flow, and oxygen transport are highly heterogeneous. This heterogeneity can strongly affect oxygen supply and organ function, including reduced oxygen uptake in the lung and decreased oxygen delivery to tissue. The causes of heterogeneity can be classified as extrinsic or intrinsic. Extrinsic heterogeneity refers to variations in oxygen demand in the systemic circulation or oxygen supply in the lungs. Intrinsic heterogeneity refers to structural heterogeneity due to stochastic growth of blood vessels and variability in flow pathways due to geometric constraints, and resulting variations in blood flow and hematocrit. Mechanisms have evolved to compensate for heterogeneity and thereby improve oxygen uptake in the lung and delivery to tissue. These mechanisms, which involve long-term structural adaptation and short-term flow regulation, depend on upstream responses conducted along vessel walls, and work to redistribute flow and maintain blood and tissue oxygenation. Mathematically, the variance of a functional quantity such as oxygen delivery that depends on two or more heterogeneous variables can be reduced if one of the underlying variables is controlled by an appropriate compensatory mechanism. Ineffective regulatory mechanisms can result in poor oxygen delivery even in the presence of adequate overall tissue perfusion. Restoration of endothelial function, and specifically conducted responses, should be considered when addressing tissue hypoxemia and organ failure in clinical settings.
Keywords: flow regulation; microvascular networks; oxygen transport; vascular remodeling.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

