SenSARS: A Low-Cost Portable Electrochemical System for Ultra-Sensitive, Near Real-Time, Diagnostics of SARS-CoV-2 Infections
- PMID: 35582002
- PMCID: PMC8843068
- DOI: 10.1109/TIM.2021.3119147
SenSARS: A Low-Cost Portable Electrochemical System for Ultra-Sensitive, Near Real-Time, Diagnostics of SARS-CoV-2 Infections
Abstract
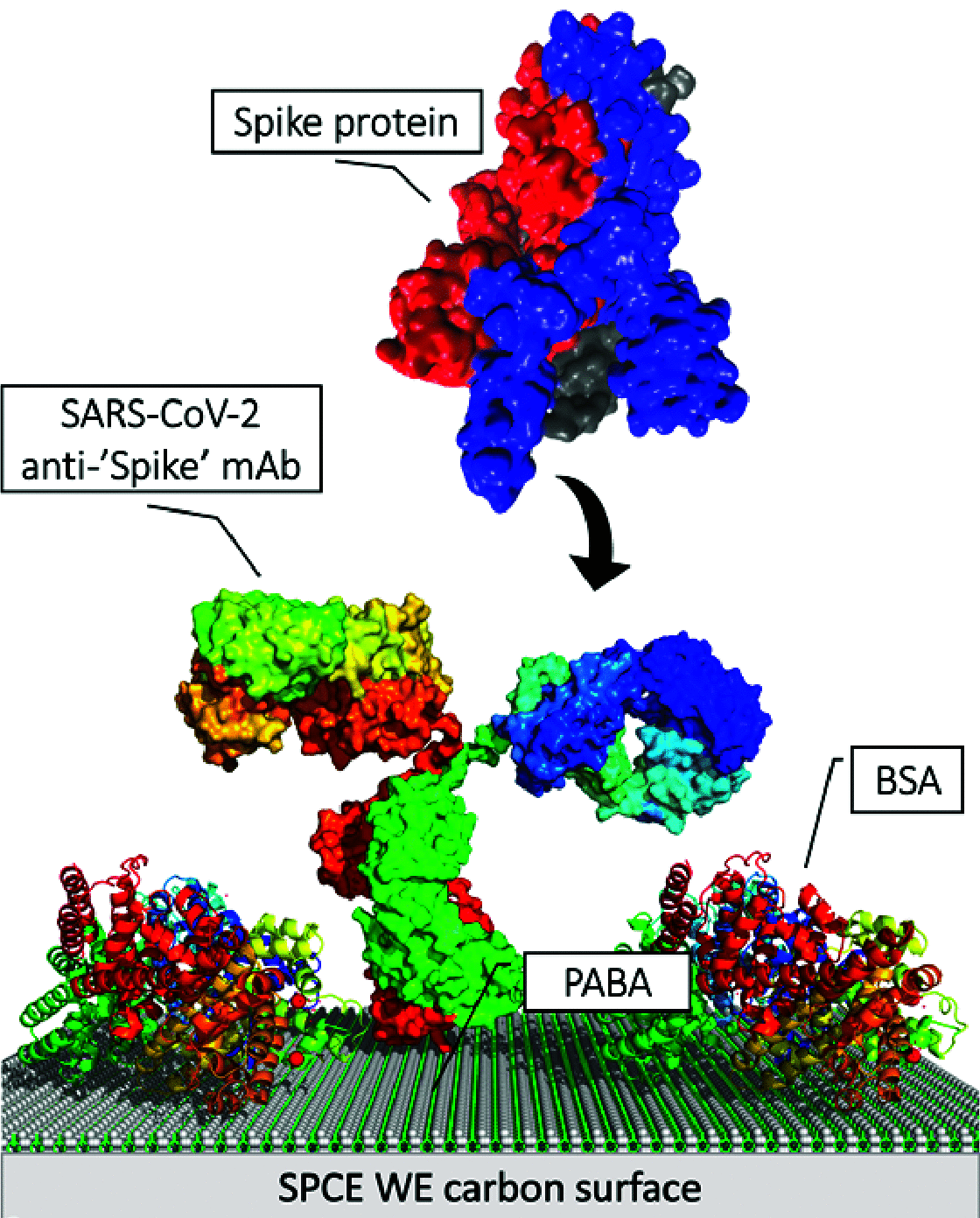
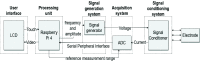
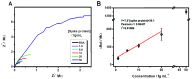
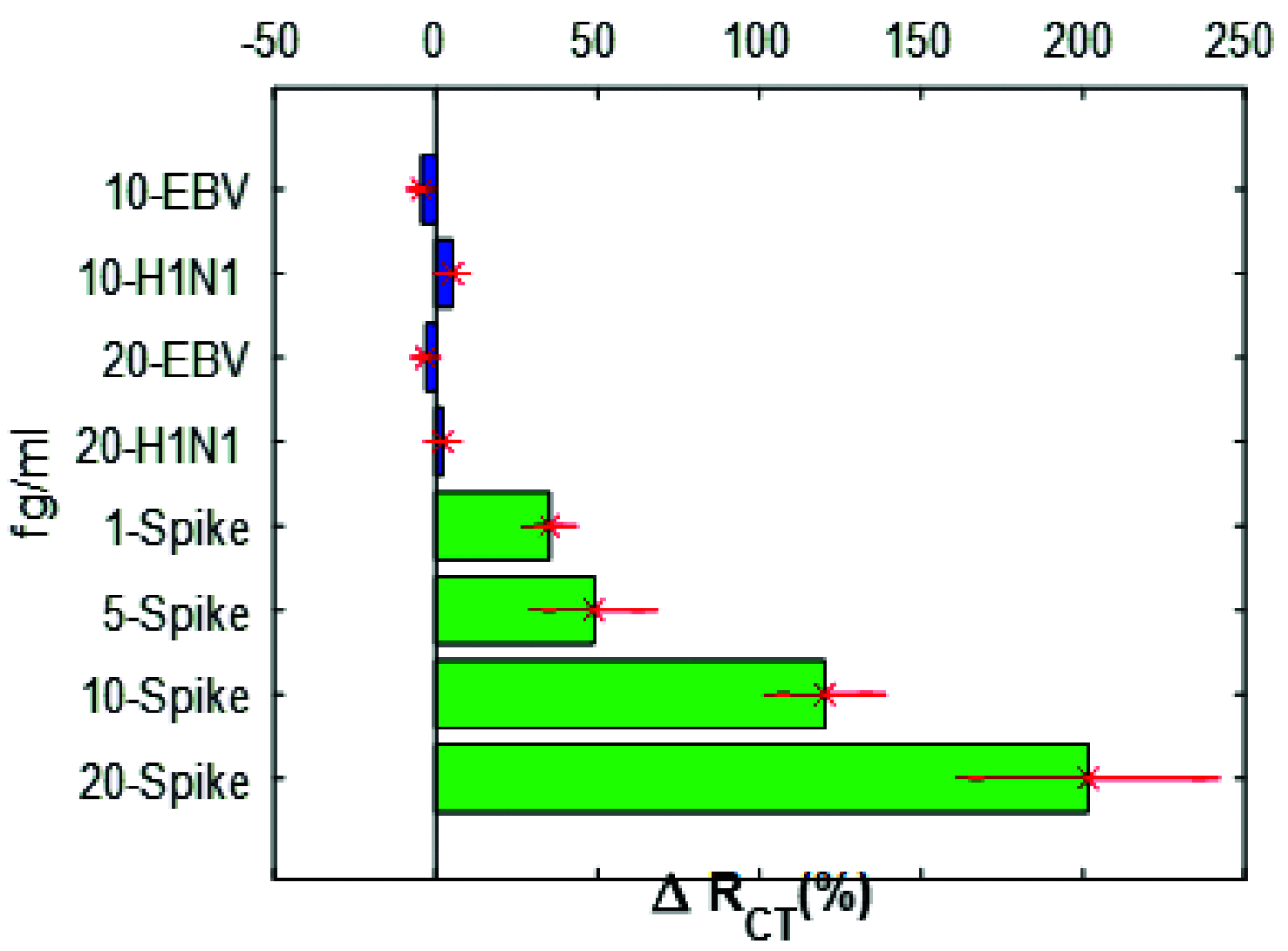
A critical path to solving the SARS-CoV-2 pandemic, without further socioeconomic impact, is to stop its spread. For this to happen, pre- or asymptomatic individuals infected with the virus need to be detected and isolated opportunely. Unfortunately, there are no current ubiquitous (i.e., ultra-sensitive, cheap, and widely available) rapid testing tools capable of early detection of SARS-CoV-2 infections. In this article, we introduce an accurate, portable, and low-cost medical device and bio-nanosensing electrode dubbed SenSARS and its experimental validation. SenSARS' device measures the electrochemical impedance spectra of a disposable bio-modified screen-printed carbon-based working electrode (SPCE) to the changes in the concentration of SARS-CoV-2 antigen molecules ("S" spike proteins) contained within a sub-microliter fluid sample deposited on its surface. SenSARS offers real-time diagnostics and viral load tracking capabilities. Positive and negative control tests were performed in phosphate-buffered saline (PBS) at different concentrations (between 1 and 50 fg/mL) of SARS-CoV-2(S), Epstein-Barr virus (EBV) glycoprotein gp350, and Influenza H1N1 M1 recombinant viral proteins. We demonstrate that SenSARS is easy to use, with a portable and lightweight (< 200 g) instrument and disposable test electrodes (<U.S. [Formula: see text]5), capable of fast diagnosis (~10 min), with high analytical sensitivity (low limits of detection, LOD = 1.065 fg/mL, and quantitation, LOQ = 3.6 fg/mL) and selectivity to SARS-CoV-2(S) antigens, even in the presence of structural proteins from the other pathogens tested. SenSARS provides a potential path to pervasive rapid diagnostics of SARS-CoV-2 in clinical, point-of-care, and home-care settings, and to breaking the transmission chain of this virus. Medical device compliance testing of SenSARS to EIC-60601 technical standards is underway.
Keywords: Biosensors; SARS-CoV-2; electrochemical biosensors; electrochemical impedance spectroscopy (EIS).
Figures



Similar articles
-
Carbon Nanostructured Immunosensing of Anti-SARS-CoV-2 S-Protein Antibodies.Molecules. 2023 Dec 9;28(24):8022. doi: 10.3390/molecules28248022. Molecules. 2023. PMID: 38138513 Free PMC article.
-
A Label-Free Electrochemical Impedimetric Immunosensor with Biotinylated-Antibody for SARS-CoV-2 Nucleoprotein Detection in Saliva.Biosensors (Basel). 2022 Apr 22;12(5):265. doi: 10.3390/bios12050265. Biosensors (Basel). 2022. PMID: 35624566 Free PMC article.
-
Disposable graphene-oxide screen-printed electrode integrated with portable device for detection of SARS-CoV-2 in clinical samples.Bioelectrochemistry. 2024 Aug;158:108722. doi: 10.1016/j.bioelechem.2024.108722. Epub 2024 Apr 27. Bioelectrochemistry. 2024. PMID: 38697015
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Electrochemical Biosensors for the Detection of SARS-CoV-2 and Other Viruses.Micromachines (Basel). 2021 Feb 10;12(2):174. doi: 10.3390/mi12020174. Micromachines (Basel). 2021. PMID: 33578979 Free PMC article. Review.
Cited by
-
Carbon-based biosensors: Next-generation diagnostic tool for target-specific detection of SARS-CoV-2 (COVID-19).Talanta Open. 2023 Aug;7:100218. doi: 10.1016/j.talo.2023.100218. Epub 2023 Apr 25. Talanta Open. 2023. PMID: 37131405 Free PMC article.
-
Polyaniline/Titania Nanotube-Based Biosensor Strip for Sensitive and Specific Electrochemical Detection of SARS-CoV-2.ACS Omega. 2023 Nov 22;8(48):45700-45707. doi: 10.1021/acsomega.3c06133. eCollection 2023 Dec 5. ACS Omega. 2023. PMID: 38075789 Free PMC article.
-
Using low-cost disposable immunosensor based on flexible PET screen-printed electrode modified with carbon black and gold nanoparticles for sensitive detection of SARS-CoV-2.Talanta Open. 2023 Aug;7:100201. doi: 10.1016/j.talo.2023.100201. Epub 2023 Mar 10. Talanta Open. 2023. PMID: 36959870 Free PMC article.
-
Detection of COVID-19-related biomarkers by electrochemical biosensors and potential for diagnosis, prognosis, and prediction of the course of the disease in the context of personalized medicine.Anal Bioanal Chem. 2023 Mar;415(6):1003-1031. doi: 10.1007/s00216-022-04237-7. Epub 2022 Aug 16. Anal Bioanal Chem. 2023. PMID: 35970970 Free PMC article. Review.
-
A Low-Cost Handheld Impedimetric Biosensing System for Rapid Diagnostics of SARS-CoV-2 Infections.IEEE Sens J. 2022 Jul 13;22(16):15673-15682. doi: 10.1109/JSEN.2022.3181580. eCollection 2022 Aug. IEEE Sens J. 2022. PMID: 36346096 Free PMC article.
References
-
- Pfefferbaum B. and North C. S., “Mental health and the COVID-19 pandemic,” New England J. Med., vol. 383, no. 6, pp. 510–512, Aug. 2020. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
