Metabolic reprogramming as an emerging mechanism of resistance to endocrine therapies in prostate cancer
- PMID: 35582011
- PMCID: PMC9019185
- DOI: 10.20517/cdr.2020.54
Metabolic reprogramming as an emerging mechanism of resistance to endocrine therapies in prostate cancer
Abstract
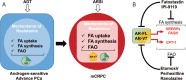
Prostate cancer (PCa) is the second leading cause of cancer-related death in the US. Androgen receptor (AR) signaling is the driver of both PCa development and progression and, thus, the major target of current in-use therapies. However, despite the survival benefit of second-generation inhibitors of AR signaling in the metastatic setting, resistance mechanisms inevitably occur. Thus, novel strategies are required to circumvent resistance occurrence and thereby to improve PCa survival. Among the key cellular processes that are regulated by androgens, metabolic reprogramming stands out because of its intricate links with cancer cell biology. In this review, we discuss how cancer metabolism and lipid metabolism in particular are regulated by androgens and contribute to the acquisition of resistance to endocrine therapy. We describe the interplay between genetic alterations, metabolic vulnerabilities and castration resistance. Since PCa cells adapt their metabolism to excess nutrient supply to promote cancer progression, we review our current knowledge on the association between diet/obesity and resistance to anti-androgen therapies. We briefly describe the metabolic symbiosis between PCa cells and tumor microenvironment and how this crosstalk might contribute to PCa progression. We discuss how tackling PCa metabolic vulnerabilities represents a potential approach of synthetic lethality to endocrine therapies. Finally, we describe how the continuous advances in analytical technologies and metabolic imaging have led to the identification of potential new prognostic and predictive biomarkers, and non-invasive approaches to monitor therapy response.
Keywords: Metabolic reprogramming; castration resistance; endocrine therapies; metabolic imaging; metabolomics; prostate cancer; therapy respo nse.
© The Author(s) 2021.
Conflict of interest statement
Chetta P is currently an employee at Boehringer-Ingelheim RCV GmbH, Vienna, Austria. The authors declared there are no conflicts of interest.
Figures

Similar articles
-
Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New Opportunities for Therapy.Int J Mol Sci. 2019 May 28;20(11):2626. doi: 10.3390/ijms20112626. Int J Mol Sci. 2019. PMID: 31142021 Free PMC article. Review.
-
Steroid hormone synthetic pathways in prostate cancer.Transl Androl Urol. 2013 Sep;2(3):212-227. doi: 10.3978/j.issn.2223-4683.2013.09.16. Transl Androl Urol. 2013. PMID: 25379460 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Lipid Metabolism and Epigenetics Crosstalk in Prostate Cancer.Nutrients. 2022 Feb 18;14(4):851. doi: 10.3390/nu14040851. Nutrients. 2022. PMID: 35215499 Free PMC article. Review.
-
Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level.Eur Urol. 2015 Mar;67(3):470-9. doi: 10.1016/j.eururo.2014.09.049. Epub 2014 Oct 8. Eur Urol. 2015. PMID: 25306226 Free PMC article. Review.
Cited by
-
Molecular Atlas of HER2+ Breast Cancer Cells Treated with Endogenous Ligands: Temporal Insights into Mechanisms of Trastuzumab Resistance.Cancers (Basel). 2024 Jan 27;16(3):553. doi: 10.3390/cancers16030553. Cancers (Basel). 2024. PMID: 38339304 Free PMC article.
-
Targeting Mitochondrial OXPHOS and Their Regulatory Signals in Prostate Cancers.Int J Mol Sci. 2021 Dec 14;22(24):13435. doi: 10.3390/ijms222413435. Int J Mol Sci. 2021. PMID: 34948229 Free PMC article. Review.
-
Prostate Cancer Progression: as a Matter of Fats.Front Oncol. 2021 Jul 27;11:719865. doi: 10.3389/fonc.2021.719865. eCollection 2021. Front Oncol. 2021. PMID: 34386430 Free PMC article. Review.
-
A Compound That Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer.Mol Cancer Ther. 2024 Jul 2;23(7):973-994. doi: 10.1158/1535-7163.MCT-23-0540. Mol Cancer Ther. 2024. PMID: 38507737 Free PMC article.
-
Evidence of the Link between Stroma Remodeling and Prostate Cancer Prognosis.Cancers (Basel). 2024 Sep 21;16(18):3215. doi: 10.3390/cancers16183215. Cancers (Basel). 2024. PMID: 39335188 Free PMC article. Review.
References
-
- Graf RP, Hullings M, Barnett ES, Carbone E, Dittamore R, Scher HI. Clinical utility of the nuclear-localized AR-V7 biomarker in circulating tumor cells in improving physician treatment choice in castration-resistant prostate cancer. Eur Urol. 2020;77:170–7. doi: 10.1016/j.eururo.2019.08.020. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
