Evaluation of the reducing potential of PSMA-containing endosomes by FRET imaging
- PMID: 35582012
- PMCID: PMC9019189
- DOI: 10.20517/cdr.2020.84
Evaluation of the reducing potential of PSMA-containing endosomes by FRET imaging
Abstract
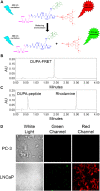
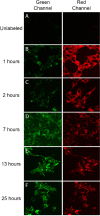
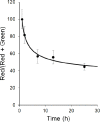
Aim: Ligand-targeted therapeutics are experiencing increasing use for treatment of human diseases due to their ability to concentrate a desired drug at a pathologic site while reducing accumulation in healthy tissues. For many ligand-targeted drug conjugates, a critical aspect of conjugate design lies in engineering release of the therapeutic payload to occur only after its internalization by targeted cells. Because disulfide bond reduction is frequently exploited to ensure intracellular drug release, an understanding of the redox properties of endocytic compartments can be critical to ligand-targeted drug design. While the redox properties of folate receptor trafficking endosomes have been previously reported, little is known about the trafficking of prostate-specific membrane antigen (PSMA), a receptor that is experiencing increasing use for drug targeting in humans. Methods: To obtain this information, we have constructed a PSMA-targeted fluorescence resonance energy transfer pair that reports on disulfide bond reduction by changing fluorescence from red to green. Results: We show here that this reporter exhibits rapid and selective uptake by PSMA-positive cells, and that reduction of its disulfide bond proceeds steadily but incompletely following internalization. The fact that maximal disulfide reduction reaches only ~50%, even after 24 h incubation, suggests that roughly half of the conjugates must traffic through endosomes that display no reducing capacity. Conclusion: As the level of disulfide reduction differs between PSMA trafficked and previously published folate trafficked conjugates, it also follows that not all internalizing receptors are translocated through similar intracellular compartments. Taken together, these data suggest that the efficiency of disulfide bond reduction must be independently analyzed for each receptor trafficking pathway when disulfide bond reduction is exploited for intracellular drug release.
Keywords: DUPA; endocytosis; endosomes; prostate-specific membrane antigen.
© The Author(s) 2021.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging.Proc Natl Acad Sci U S A. 2006 Sep 12;103(37):13872-7. doi: 10.1073/pnas.0601455103. Epub 2006 Sep 1. Proc Natl Acad Sci U S A. 2006. PMID: 16950881 Free PMC article.
-
Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates.Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17987-92. doi: 10.1073/pnas.0509035102. Epub 2005 Dec 1. Proc Natl Acad Sci U S A. 2005. PMID: 16322102 Free PMC article.
-
Prostate-Specific Membrane Antigen Targeted Therapy of Prostate Cancer Using a DUPA-Paclitaxel Conjugate.Mol Pharm. 2018 May 7;15(5):1842-1852. doi: 10.1021/acs.molpharmaceut.8b00026. Epub 2018 Apr 5. Mol Pharm. 2018. PMID: 29608845
-
New Prostate Cancer Targets for Diagnosis, Imaging, and Therapy: Focus on Prostate-Specific Membrane Antigen.Front Oncol. 2018 Dec 21;8:653. doi: 10.3389/fonc.2018.00653. eCollection 2018. Front Oncol. 2018. PMID: 30622933 Free PMC article. Review.
-
Disulfide-containing parenteral delivery systems and their redox-biological fate.J Control Release. 2014 Dec 10;195:147-54. doi: 10.1016/j.jconrel.2014.06.012. Epub 2014 Jun 18. J Control Release. 2014. PMID: 24952369 Review.
Cited by
-
Selective targeting of chemically modified miR-34a to prostate cancer using a small molecule ligand and an endosomal escape agent.Mol Ther Nucleic Acids. 2024 Apr 23;35(2):102193. doi: 10.1016/j.omtn.2024.102193. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38745855 Free PMC article.
-
Recent advances in tumor-targeting chemotherapy drugs.Cancer Drug Resist. 2021;4(4):885-887. doi: 10.20517/cdr.2021.86. Epub 2021 Sep 8. Cancer Drug Resist. 2021. PMID: 35309517 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
