Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway
- PMID: 35582102
- PMCID: PMC9048534
- DOI: 10.4251/wjgo.v14.i4.872
Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway
Abstract
Background: The phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signalling pathway is crucial for cell survival, differentiation, apoptosis and metabolism. Xihuang pills (XHP) are a traditional Chinese preparation with antitumour properties. They inhibit the growth of breast cancer, glioma, and other tumours by regulating the PI3K/Akt/mTOR signalling pathway. However, the effects and mechanisms of action of XHP in hepatocellular carcinoma (HCC) remain unclear. Regulation of the PI3K/Akt/mTOR signalling pathway effectively inhibits the progression of HCC. However, no study has focused on the XHP-associated PI3K/Akt/mTOR signalling pathway. Therefore, we hypothesized that XHP might play a role in inhibiting HCC through the PI3K/Akt/mTOR signalling pathway.
Aim: To confirm the effect of XHP on HCC and the possible mechanisms involved.
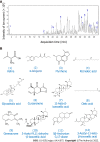
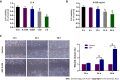
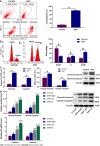
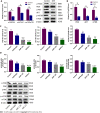
Methods: The chemical constituents and active components of XHP were analysed using ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (UPLC-Q-TOF-MS). Cell-based experiments and in vivo xenograft tumour experiments were utilized to evaluate the effect of XHP on HCC tumorigenesis. First, SMMC-7721 cells were incubated with different concentrations of XHP (0, 0.3125, 0.625, 1.25, and 2.5 mg/mL) for 12 h, 24 h and 48 h. Cell viability was assessed using the CCK-8 assay, followed by an assessment of cell migration using a wound healing assay. Second, the effect of XHP on the apoptosis of SMMC-7721 cells was evaluated. SMMC-7721 cells were stained with fluorescein isothiocyanate and annexin V/propidium iodide. The number of apoptotic cells and cell cycle distribution were measured using flow cytometry. The cleaved protein and mRNA expression levels of caspase-3 and caspase-9 were detected using Western blotting and quantitative reverse-transcription polymerase chain reaction (RT-qPCR), respectively. Third, Western blotting and RT-qPCR were performed to confirm the effects of XHP on the protein and mRNA expression of components of the PI3K/Akt/mTOR signalling pathway. Finally, the effects of XHP on the tumorigenesis of subcutaneous hepatocellular tumours in nude mice were assessed.
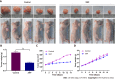
Results: The following 12 compounds were identified in XHP using high-resolution mass spectrometry: Valine, 4-gingerol, myrrhone, ricinoleic acid, glycocholic acid, curzerenone, 11-keto-β-boswellic acid, oleic acid, germacrone, 3-acetyl-9,11-dehydro-β-boswellic acid, 5β-androstane-3,17-dione, and 3-acetyl-11-keto-β-boswellic acid. The cell viability assay results showed that treatment with 0.625 mg/mL XHP extract decreased HCC cell viability after 12 h, and the effects were dose- and time-dependent. The results of the cell scratch assay showed that the migration of HCC cells was significantly inhibited in a time-dependent manner by the administration of XHP extract (0.625 mg/mL). Moreover, XHP significantly inhibited cell migration and resulted in cell cycle arrest and apoptosis. Furthermore, XHP downregulated the PI3K/Akt/mTOR signalling pathway, which activated apoptosis executioner proteins (e.g., caspase-9 and caspase-3). The inhibitory effects of XHP on HCC cell growth were determined in vivo by analysing the tumour xenograft volumes and weights.
Conclusion: XHP inhibited HCC cell growth and migration by stimulating apoptosis via the downregulation of the PI3K/Akt/mTOR signalling pathway, followed by the activation of caspase-9 and caspase-3. Our findings clarified that the antitumour effects of XHP on HCC cells are mediated by the PI3K/Akt/mTOR signalling pathway, revealing that XHP may be a potential complementary therapy for HCC.
Keywords: Antitumour; Apoptosis; Hepatocellular carcinoma; Phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway; Xihuang pills.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no competing interests.
Figures
Similar articles
-
Exploring the Pharmacological Mechanisms of Xihuang Pills Against Prostate Cancer via Integrating Network Pharmacology and Experimental Validation In Vitro and In Vivo.Front Pharmacol. 2022 Mar 7;12:791269. doi: 10.3389/fphar.2021.791269. eCollection 2021. Front Pharmacol. 2022. PMID: 35342388 Free PMC article.
-
[Xihuang Pills and its main components inhibit PI3K/Akt/mTOR signaling pathways and promote the apoptosis of prostate cancer cells in PC-3 tumor-bearing mice].Zhonghua Nan Ke Xue. 2021 Apr;27(4):340-346. Zhonghua Nan Ke Xue. 2021. PMID: 34914218 Chinese.
-
Xihuang Pill-destabilized CD133/EGFR/Akt/mTOR cascade reduces stemness enrichment of glioblastoma via the down-regulation of SOX2.Phytomedicine. 2023 Jun;114:154764. doi: 10.1016/j.phymed.2023.154764. Epub 2023 Mar 16. Phytomedicine. 2023. PMID: 36963368
-
PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review).Exp Ther Med. 2021 May;21(5):540. doi: 10.3892/etm.2021.9972. Epub 2021 Mar 23. Exp Ther Med. 2021. PMID: 33815613 Free PMC article. Review.
-
Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours.Cell Biosci. 2020 Apr 1;10(1):54. doi: 10.1186/s13578-020-00416-0. eCollection 2020. Cell Biosci. 2020. Retraction in: Cell Biosci. 2021 Aug 06;11(1):157. doi: 10.1186/s13578-021-00667-5. PMID: 32266056 Free PMC article. Retracted. Review.
Cited by
-
Anti-tumor Effects of Pollen Pini on Hepatocarcinoma SMMC-7721 Cells via Modulation of PI3K-Akt Signaling Pathway.Chin J Integr Med. 2025 Aug 15. doi: 10.1007/s11655-025-4132-2. Online ahead of print. Chin J Integr Med. 2025. PMID: 40813555
-
Xingxiao Pill Suppressed the Progression of Non-Small Cell Lung Cancer by Targeting SREBP1/FASN-Induced Fatty Acid Biosynthesis via PI3K/AKT/mTOR Signaling Pathway.Cancer Manag Res. 2025 Jul 24;17:1487-1501. doi: 10.2147/CMAR.S510010. eCollection 2025. Cancer Manag Res. 2025. PMID: 40734684 Free PMC article.
-
Integrating Chinese medicine into mainstream cancer therapies: a promising future.Front Oncol. 2024 Jun 18;14:1412370. doi: 10.3389/fonc.2024.1412370. eCollection 2024. Front Oncol. 2024. PMID: 38957318 Free PMC article. Review.
-
Meta-analysis of the efficacy and safety of Xihuang Pills/capsules in adjuvant treatment of uterine cervical neoplasms.Medicine (Baltimore). 2023 Aug 25;102(34):e34846. doi: 10.1097/MD.0000000000034846. Medicine (Baltimore). 2023. PMID: 37653807 Free PMC article.
-
Reevaluating Calculus bovis: Modulating the liver cancer immune microenvironment via the Wnt/β-catenin pathway.World J Gastroenterol. 2025 Feb 14;31(6):99750. doi: 10.3748/wjg.v31.i6.99750. World J Gastroenterol. 2025. PMID: 39958448 Free PMC article.
References
-
- Rahmani F, Ziaeemehr A, Shahidsales S, Gharib M, Khazaei M, Ferns GA, Ryzhikov M, Avan A, Hassanian SM. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J Cell Physiol. 2020;235:4146–4152. - PubMed
-
- Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167. - PubMed
-
- Brotelle T, Bay JO. PI3K-AKT-mTOR pathway: Description, therapeutic development, resistance, predictive/prognostic biomarkers and therapeutic applications for cancer. Bull Cancer. 2016;103:18–29. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

