Bi-specific T1 positive-contrast-enhanced magnetic resonance imaging molecular probe for hepatocellular carcinoma in an orthotopic mouse model
- PMID: 35582105
- PMCID: PMC9048532
- DOI: 10.4251/wjgo.v14.i4.858
Bi-specific T1 positive-contrast-enhanced magnetic resonance imaging molecular probe for hepatocellular carcinoma in an orthotopic mouse model
Abstract
Background: Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality. HCC-targeted magnetic resonance imaging (MRI) is an effective noninvasive diagnostic method that involves targeting clinically-related HCC biomarkers, such as alpha-fetoprotein (AFP) or glypican-3 (GPC3), with iron oxide nanoparticles. However, in vivo studies of HCC-targeted MRI utilize single-target iron oxide nanoprobes as negative (T2) contrast agents, which might weaken their future clinical applications due to tumor heterogeneity and negative MRI contrast. Ultra-small superparamagnetic iron oxide (USPIO) nanoparticles (approximately 5 nm) are potential optimal positive (T1) contrast agents. We previously verified the efficiency of AFP/GPC3-double-antibody-labeled iron oxide MR molecular probe in vitro.
Aim: To validate the effectiveness of a bi-specific probe in vivo for enhancing T1-weighted positive contrast to diagnose the early-stage HCC.
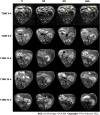
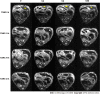
Methods: The single- and double-antibody-conjugated 5-nm USPIO probes, including anti-AFP-USPIO (UA), anti-GPC3-USPIO (UG), and anti-AFP-USPIO-anti-GPC3 (UAG), were synthesized. T1- and T2-weighted MRI were performed on day 10 after establishment of the orthotopic HCC mouse model. Following intravenous injection of U, UA, UG, and UAG probes, T1- and T2-weighted images were obtained at 12, 12, and 32 h post-injection. At the end of scanning, mice were euthanized, and a histologic analysis was performed on tumor samples.
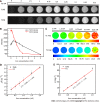
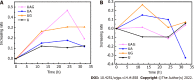
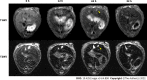
Results: T1- and T2-weighted MRI showed that absolute tumor-to-background ratios in UAG-treated HCC mice peaked at 24 h post-injection, with the T1- and T2-weighted signals increasing by 46.7% and decreasing by 11.1%, respectively, relative to pre-injection levels. Additionally, T1-weighted contrast in the UAG-treated group at 24 h post-injection was enhanced 1.52-, 2.64-, and 4.38-fold compared to those observed for single-targeted anti-GPC3-USPIO, anti-AFP-USPIO, and non-targeted USPIO probes, respectively. Comparison of U-, UA-, UG-, and UAG-treated tumor sections revealed that UAG-treated mice exhibited increased stained regions compared to those observed in UG- or UA-treated mice.
Conclusion: The bi-specific T1-positive contrast-enhanced MRI probe (UAG) for HCC demonstrated increased specificity and sensitivity to diagnose early-stage HCC irrespective of tumor size and/or heterogeneity.
Keywords: Alpha-fetoprotein; Glypican-3; Hepatocellular carcinoma; Magnetic resonance imaging; Molecular imaging; Positive contrast agent.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that there are no conflicts of interest regarding the publication of the paper.
Figures


Similar articles
-
Development and in vitro study of a bi-specific magnetic resonance imaging molecular probe for hepatocellular carcinoma.World J Gastroenterol. 2019 Jun 28;25(24):3030-3043. doi: 10.3748/wjg.v25.i24.3030. World J Gastroenterol. 2019. PMID: 31293339 Free PMC article.
-
Preparation of magnetic resonance probes using one-pot method for detection of hepatocellular carcinoma.World J Gastroenterol. 2015 Apr 14;21(14):4275-83. doi: 10.3748/wjg.v21.i14.4275. World J Gastroenterol. 2015. PMID: 25892879 Free PMC article.
-
A GPC3-specific aptamer-mediated magnetic resonance probe for hepatocellular carcinoma.Int J Nanomedicine. 2018 Aug 1;13:4433-4443. doi: 10.2147/IJN.S168268. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30122918 Free PMC article.
-
Ovarian cancer antigen 183B2 monoclonal antibody conjugated to ultrasmall superparamagnetic iron oxide nanoparticles.2011 Jan 5 [updated 2011 Apr 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 5 [updated 2011 Apr 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21510040 Free Books & Documents. Review.
-
Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate.2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Oct 13 [updated 2009 Nov 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641831 Free Books & Documents. Review.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Yang D, She H, Wang X, Yang Z, Wang Z. Diagnostic accuracy of quantitative diffusion parameters in the pathological grading of hepatocellular carcinoma: A meta-analysis. J Magn Reson Imaging. 2020;51:1581–1593. - PubMed
-
- Semaan S, Vietti Violi N, Lewis S, Chatterji M, Song C, Besa C, Babb JS, Fiel MI, Schwartz M, Thung S, Sirlin CB, Taouli B. Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur Radiol. 2020;30:1020–1030. - PubMed
LinkOut - more resources
Full Text Sources

